ChemInform Abstract: PPL-Catalyzed Resolution of 1,2- and 1,3-Diols in Methyl Propionate as Solvent. An Application of the Tandem Use of Enzymes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
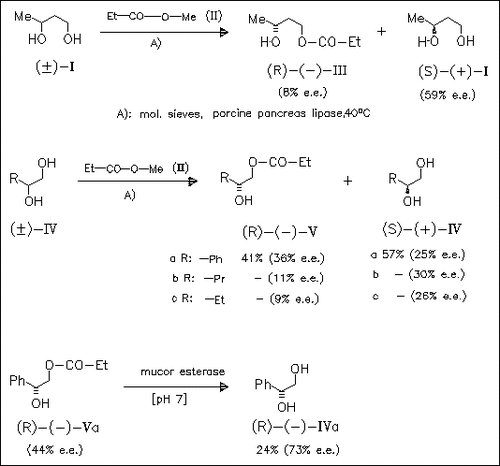
In the presence of PPL (porcine pancreas lipase) the 1,2-diols (IV) and the 1,3-diol (I) are esterified by methyl propionate (II) exclusively at the primary alcohol function to afford the monoesters ( III) and (V) along with the (S) diols (I) and (IV). The enantiomeric excesses of the monoesters which are determined via alkaline hydrolysis to the corresponding (R) diols are only low to moderate. The enantiomeric excess of the (R) diol is enhanced when the monoesters are submitted to enzymatically catalyzed hydrolysis. Thus, the monoester (R)-(Va) is hydrolysed in the presence of mucor esterase to give the (R) diol (R)-(IVa) with 73% e.e. via this “tandem” procedure.