ChemInform Abstract: An Enantioselective Construction of Pyrrolidones Bearing Functionalized Appendages via an Asymmetric Intramolecular Michael Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
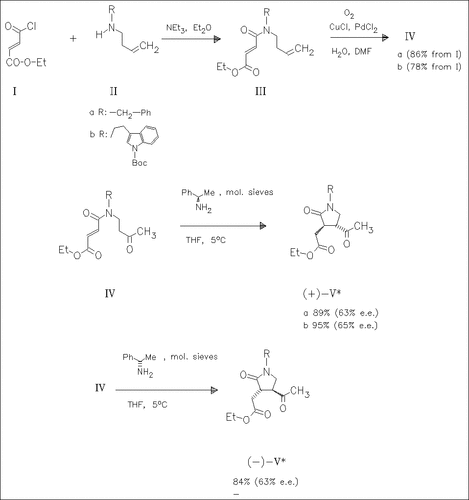
Acylation of the homoallylamines (II) with the fumaric monochloride (I) in the presence of triethylamine yields the unsaturated carboxamides ( III) which are oxidized with oxygen, forming the methyl ketones (IV). Treatment of the latter with (R)-(+)-1-phenylethylamine or (S)-(-)-1- phenylethylamine results in intramolecular cyclization to produce the oxopyrrolidineacetates (+)-(V) or (-)-(V).