ChemInform Abstract: Stereocontrolled Epoxidations of Cycloheptene Derivatives in the Palladium-Catalyzed Route to Tropane Alkaloids. Total Syntheses of Scopine and Pseudoscopine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
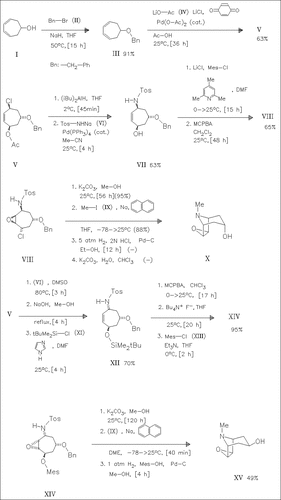
Benzylation of the cycloheptadienol (I) followed by palladium- catalyzed 1,4-chloroacetoxylation yields stereoselectively the cycloheptene derivative (V). Reductive deacetylation of (V) and palladium-catalyzed coupling with sodium tosylamide (VI) form the N- tosylamino alcohol (VII) which is converted to the epoxide (VIII) and then consecutively cyclized, methylated, and debenzylated to produce the alkaloid scopine (X). Nucleophilic substitution reaction of (V) with the compound (VI) followed by deacetylation and silylation yields the tosylaminocycloheptene derivative (XII) which is converted to pseudoscopine (XV).