ChemInform Abstract: Synthesis of 5-Methyl-6H-pyrido(4,3-b)carbazole-11-methanol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
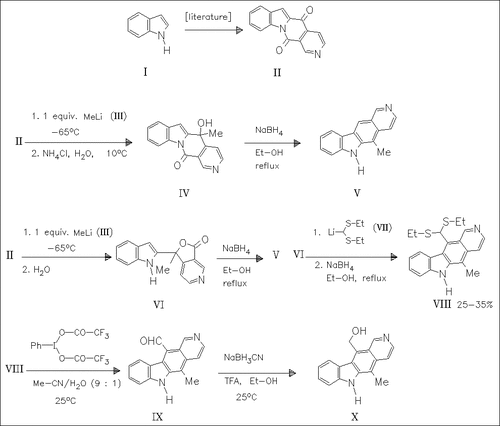
The keto lactam (II), prepared from indole (I) in five steps, is reductively methylated with methyllithium (III) to yield either the hydroxylactam (IV) or the lactone (VI) depending on the work-up procedure. Both, (IV) and (VI) undergo rearrangement upon further reduction, forming 11-porellipticine (V). The lactone (VI) and the trimethylsilyl ether derived from (IV) are coupled with lithiated formaldehyde diethyl dithio acetal (VII) and then reductively rearranged to produce the pyridocarbazole (VIII). Degradation of the thioacetal moiety, followed by reduction of the aldehyde group, gives the title compound (X). (Yields only partly given).