ChemInform Abstract: Structure-Affinity Relationships of 12-Sulfonyl Derivatives of 5,8,8a, 9,10,11,12,12a,13,13a-Decahydro-6H-isoquino(2,1-g)(1,6)naphthyridines at α-Adrenoceptors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
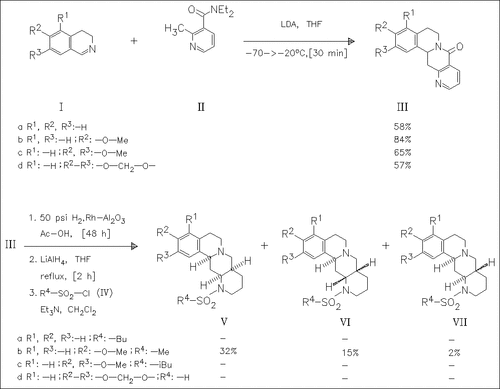
LDA-mediated coupling of the dihydroisoquinolines (I) with the nicotinamide (II) forms the tetrahydroisoquinonaphthyridinones (III). Catalytical hydrogenation followed by reduction of the lactam and reaction with sulfonyl chlorides such as (IV) yields the diastereomeric decahydroisoquino(1,6)naphthyridines (V) - (VII) mentioned in the title. The derivative (Vb) is found to be a potent . alpha.2-adrenoceptor antagonist in vivo. Yields are only partly given.