ChemInform Abstract: A Route to 1,1-Diorganometallic Species via the Claisen Rearrangement of Functionalized γ-Hydroxyvinylstannanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
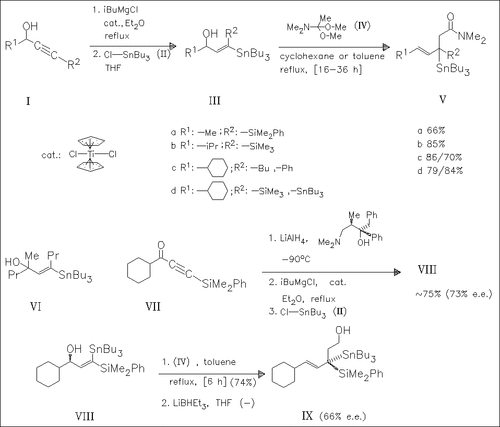
The propargylic alcohols (I) are converted to the vinylic stannanes (III) by a known procedure. Reaction of (III) with dimethylacetamide dimethyl acetal (IV) forms mainly the trans-configurated γ,δ-unsaturated amides (V). The carbinol (VI) does not react under these conditions. The silylalkynone (VII) is transformed into the chiral stannane (VIII) which is consecutively coupled with (IV) and reduced, yielding the optically active alcohol (IX).