ChemInform Abstract: Intramolecular Participation of the Amide Group in Acid- and Base- Catalysed Phosphonate Monoester Hydrolysis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
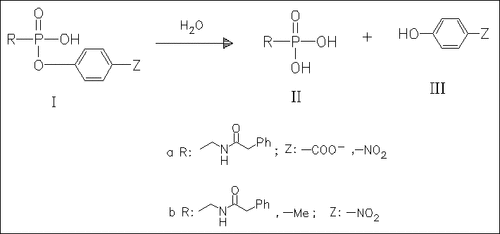
The hydrolysis of phosphonates such as (I) shows an acceleration in both acidic and alkaline solution, in the presence of an acylamido group in (Ia) in contrast to (Ib). It is assumed that intramolecular amide groups participate in the reaction, leading to a five-membered cyclic intermediate. This phenomenon is important in enzyme inhibition.