ChemInform Abstract: Organoselenium- and Proton-Mediated Cyclization Reactions of Allylic Amides and Thioamides. Syntheses of 2-Oxazolines and 2-Thiazolines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
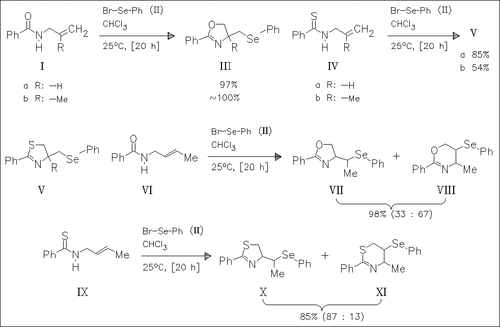
Treatment of the allylic amides (I), (VI), or thioamides (IV) and (IX) with phenylselenenyl bromide (II) results in 5-exo cyclization, forming the 2-oxazolines (III), (VII), or 2-thiazolines (V) and (X). In some cases, the dihydro-1,3-oxazines (VIII) or the corresponding thiazines (XI) are formed as additional products via 6-endo-ring closure.