ChemInform Abstract: Synthesis and Properties of New π-Donor Sulfur Heterocycles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
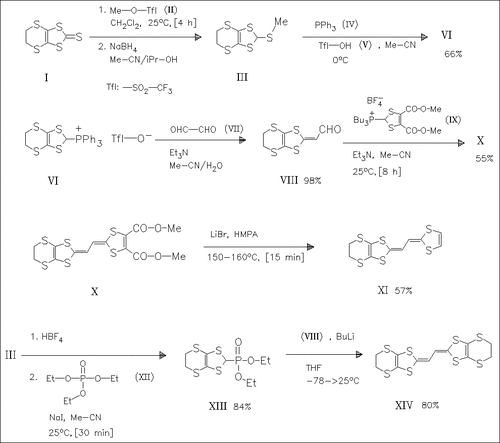
Reaction of the thione (I) with methyl triflate (II) followed by boranate reduction yields the trithioorthoformate (III) which is successively coupled with triphenylphosphine (IV) and glyoxal (VII), forming the aldehyde (VIII). This reacts with the phosphonium salt (IX) or the phosphonate (XIII) to give the ethanediylidene-2,2′-bis(1,3- dithioles) (X) and (XIV) respectively. Decarbomethoxylation of (X) by means of lithium bromide in HMPA produces the parent compound (XI).