ChemInform Abstract: Muscarinic Cholinergic Agonists and Antagonists of the 3-(3-Alkyl-1,2, 4-oxadiazol-5-yl)-1,2,5,6-tetrahydropyridine Type. Synthesis and Structure-Activity Relationships.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
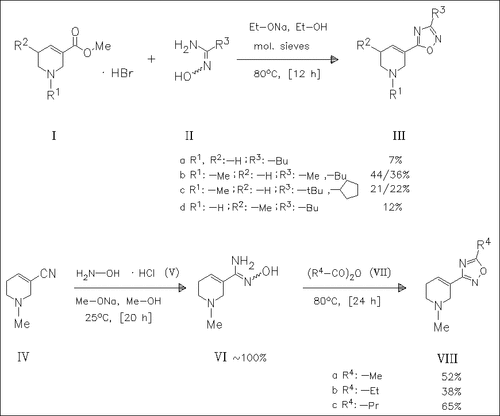
Reaction of the tetrahydro-3-pyridinecarboxylates (I) with amidoximes such as (II) forms the corresponding oxadiazolyl derivatives (III). The isomers (VIII) are prepared by reaction of the nitrile (IV) with hydroxylamine hydrochloride (V) followed by cyclization with the carboxylic anhydrides (VII). In biological tests mentioned in the title, the branched or cyclic derivatives (IIIc) are antagonists, whereas the unbranched compounds, e.g. (IIIb), are agonists.