ChemInform Abstract: Synthesis and Protein-Tyrosine Kinase Inhibitory Activities of Flavonoid Analogues.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
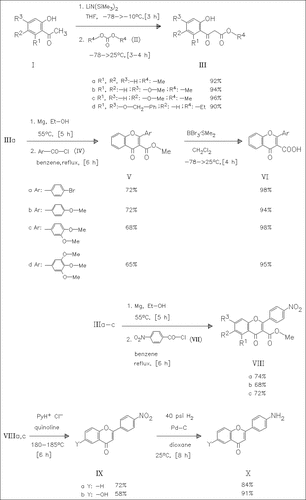
Reaction of the o-hydroxyacetophenones (I) with the dialkyl carbonates (II) yields the β-keto esters (III) which are converted into the magnesium chelates and then coupled with aroyl chlorides such as (IV) or (VII) to afford the corresponding oxobenzopyrancarboxylates (V) or ( VIII). Deesterification of (V) yields the carboxylic acids (VI). Treatment of (VIIIa) and (VIIIc) with pyridinium chloride in quinoline gives the nitrophenylbenzopyranones (IX) which are hydrogenated to produce the amino derivatives (X). Several further reactions are described in the original paper. The derivative (Xc) is the best inhibitor of protein-tyrosine kinase in this series.