ChemInform Abstract: Direct Conversion of N-Alkoxy β-Lactams to Carbapenams: Application to the Synthesis of the Bicyclic PS-5 Keto Ester.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
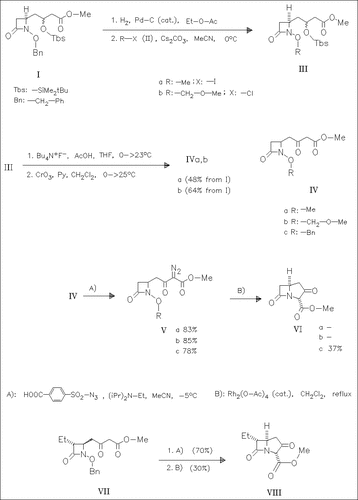
The N-alkoxy β-lactams (IVa) and (IVb), prepared as outlined in the reaction scheme, as well as the literature-known derivative (IVc) react with a sulfonyl azide according to A) to give the α-diazo β-keto esters (V). These are cyclized via carbene intermediates, forming the bicyclic β-lactams (VI). This synthetic route is applied to the synthesis of the bicyclic antibiotic PS-5 keto ester (VIII) mentioned in the title.