ChemInform Abstract: Synthesis of α-Halocinnamate Esters via Solvolytic Rearrangement of Trichloroallyl Alcohols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
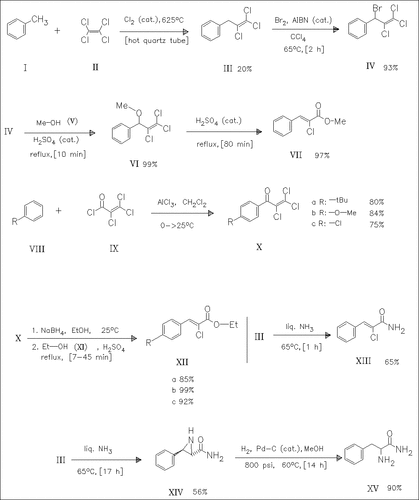
The high temperature, gas-phase coupling of toluene with perchloroethylene (II) yields the phenyltrichloropropene (III). Selective bromination and subsequent acid-catalyzed methanolysis produce the α-chlorocinnamate (VII) via the intermediate methoxy derivative (VI). A second route to substituted cinnamates such as (XII) starts with the Friedel—Crafts acylation of the substituted benzenes (VIII) followed by NaBH4 reduction and acid-catalyzed rearrangement/methanolysis. The α-chlorocinnamate (III) is converted to the aziridine (XIV) which is hydrogenated to form phenylalanine amide (XV).