ChemInform Abstract: Catalytic Oligomerization of Terminal Alkynes by Lanthanoid Carbyls (. eta.5-C5Me5)2LnCH(SiMe3)2 (Ln: Y, La, Ce).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
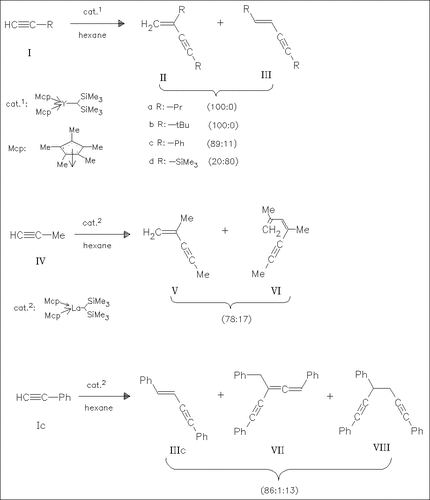
The title compounds are active catalyst precursors for the title reactions. The regioselectivity and the extent of the oligomerization depend strongly on the used lanthanoid as well as on the alkyne substituent R. For yttrium, only the dimeric products (II) and (III) are obtained. Nearly exclusively the dimers (IIa) and (IIb) are obtained from reactions catalyzed by the La and Ce title compounds. The La and Ce catalyzed oligomerization of the alkynes (IV), (Ic), and (Id) produce, besides dimers, higher oligomers (trimers, tetramers) of various sorts. A catalytic cycle involving ((η5-C5Me5)2Ln-C. tplbond.CR)n is discussed. The oligomeric Ce acetylides ((η5-C5Me5) 2Ce-CCR)n (R: tBu, Me; yield 23/18%) are synthesized from (. eta.5-C5Me5)2CeCH(SiMe3)2 and excess of alkyne.