ChemInform Abstract: Tetrahydropyridyloxadiazoles: Semirigid Muscarinic Ligands.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
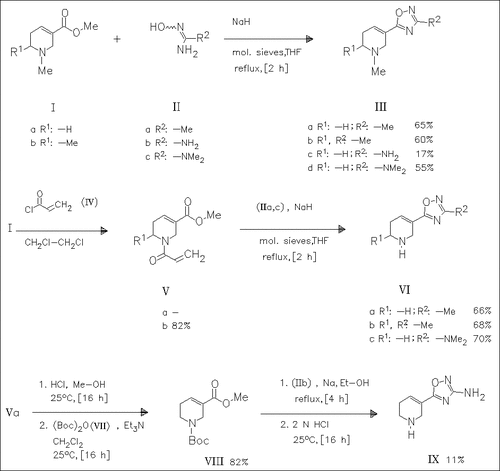
Arecoline (Ia) and 6-methylarecoline (Ib) react with the amidoximes (II) to give the oxadiazoles (III). Conversion of (I) to the N-protected derivatives (V) followed by coupling with (IIa) or (IIc) and subsequent deprotection yields the NH analogues (VI). Similarly, (IX) is synthesized as shown in the reaction scheme. The derivatives (VIa) and (IX) are the most potent muscarinic antagonists among the compounds prepared.