ChemInform Abstract: Smooth Generation of 3H-2-Benzopyran-3-ones and Their Diels-Alder Reactions with Olefinic Dienophiles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
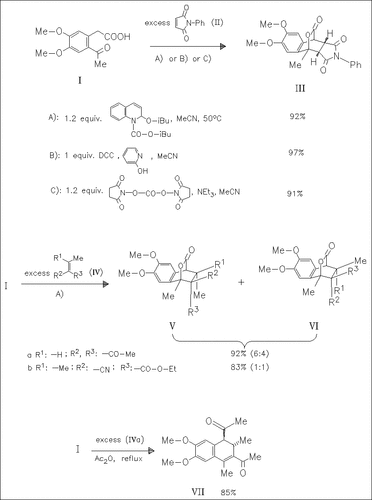
It is shown that DCC, disuccinimidyl carbonate and 2-isobutoxy-N-isobutoxycarbonyl-1,2-dihydroquinoline (IIDQ) are potent reagents to synthesize benzopyranones. These unstable intermediates are trapped with dienophiles to give the corresponding Diels—Alder adducts. The use of IIDQ opens new perspectives for Diels—Alder reactions of unstable and less reactive dienophiles with problematic benzopyranones. In contrast, the acetic anhydride method is found to be inefficient when acids of type (I) are used as starting materials. In these cases loss of CO2 or decomposition are observed.