ChemInform Abstract: Acylketene Acetals in Organic Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
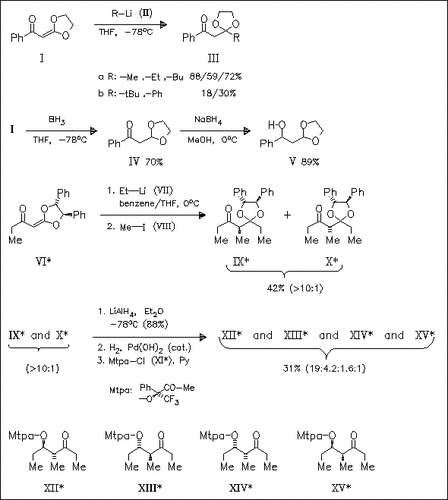
Acylketene acetals such as (I) undergo facile organolithium addition (. fwdarw. (III)) and conjugate hydroboration reaction (→ (IV)). The enantiomerically pure acylketene acetal (VI) is employed in the synthesis of the stereoisomers (XII)-(XV) of the Mtpa-ester of the insect pheromone sitophilure (XV).