ChemInform Abstract: Stereospecific Cyclization of Vinyl Ether Alcohols. Facile Synthesis of (-)-Lardolure.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
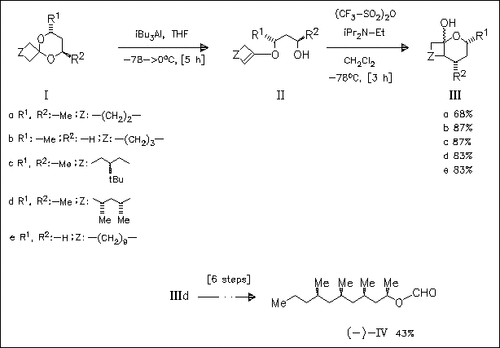
Acetals such as (I) react with triisobutylaluminum to form the elimination prdoucts (II) which are treated with trifluoromethanesulfonic acid anhydride to prepare the cyclic hemiacetals (III). The derivative (IIId) is converted in a six-step synthesis into (-)-lardolure (IV).