ChemInform Abstract: Fluorine- and Trifluoromethyl-Substituted Toluenes: Site Selective Metalation of Aromatic or Benzylic Positions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
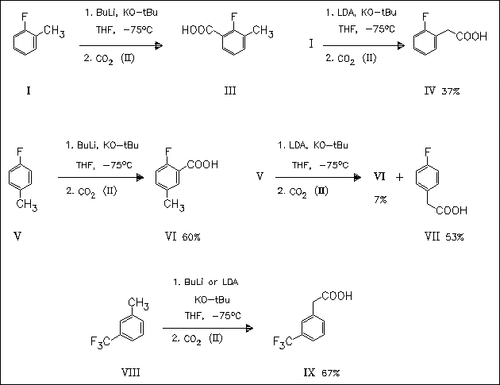
Fluorotoluenes, e.g. (I) and (V), react with a mixture of butyllithium and potassium tert-butoxide under exclusive metalation at an aromatic position adjacent to the halogen, as demonstrated by quenching with carbon dioxide (II) to give the corresponding benzoic acids, e.g. (III) and (VI). In contrast, a mixture of lithium diisopropylamide and potassium tert-butoxide selectively deprotonates the benzylic positions of fluorotoluenes to give the corresponding phenylacetic acids after quenching with carbon dioxide (II). The contrasting behavior is explained on the basis of the different reaction energy profiles. With 3-(trifluoromethyl)toluene (IV), metal/hydrogen exchange again occurs at the methyl group independently of the lithium agent employed while the corresponding 2- and 4-isomers do not produce interceptable organometallic intermediates.