ChemInform Abstract: Synthesis of Conformationally Restricted Chiral γ-Aminobutyric Acid (GABA) Analogues.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
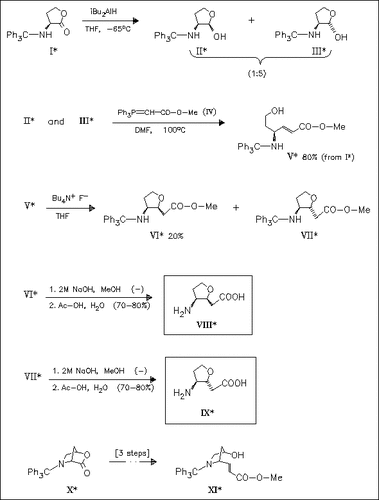
The title compounds (VIII) and (IX) are prepared from the homoserine lactone (I). The analogous preparation of a bicyclic analogue from the hydroxyproline lactone (X) is unsuccessful, because in the last reaction step the ester (XI) cannot be cyclized (yield of (VII) given in g).