ChemInform Abstract: Synthesis and Conversions of Some Dienes with a Caryophyllane Skeleton in Acidic Media.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
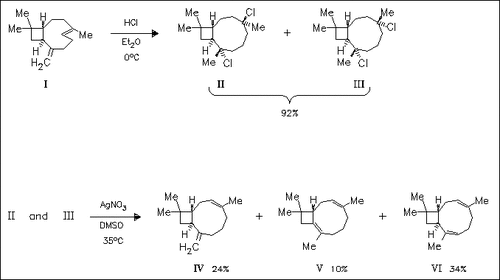
Hydrochlorination of caryophyllene (I) affords a mixture of the isomeric derivatives (II) and (III). Dehydrochlorination yields the hitherto unknown title compounds (IV), (V) and (VI); their chemical conversions in media of varying acidity are investigated.