ChemInform Abstract: Synthesis, Antiproliferative, and Antiviral Activity of Certain 4- Substituted and 3,5-Disubstituted 7-((1,3-Dihydroxy-2-propoxy)methyl) pyrrolo(2,3-d)pyrimidines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
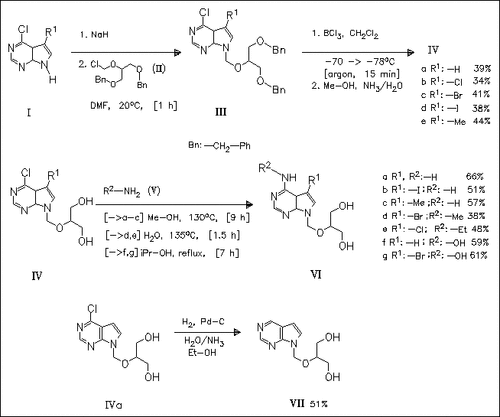
Coupling of the sodium salts of the pyrrolo(2,3-d)pyrimidines (I) with (1,3-bis(benzyloxy)-2-propoxy)methyl chloride (II) produces the derivatives (III) which are debenzylated with boron trichloride to yield the pyrrolo(2,3-d)pyrimidines (IV). Condensation reaction of (IV) with the amines (V) forms the title compounds (VI). The nebularine analogue (VII) is obtained by hydrogenation of (IVa). The compounds ( VI) have antiproliferative and antiviral activity.