ChemInform Abstract: Benzoxathiete and Related Structures: Experimental and Quantum Chemical Studies.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
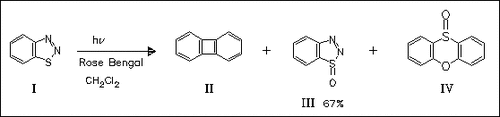
In continuation of earlier work, the aprotic diazotization of 2-(2- acetoxyethylsulfinyl) (and sulfonyl)anilines with isoamyl nitrite is reported. Attempts are described to evaluate eventually operating mechanisms, e.g., by using spin traps such as 5,5-dimethyl-1-pyrroline N-oxide. A wide range of calculations is performed on plausible radicalic intermediates and intermediate products such as benzoxathiete or dehydrobenzene. The S-oxide (III) is synthesized chemically (30% H2O2 in 1:1 MeOH/AcOH, 45 d room temp.; 60% yield) and also photochemically (see scheme). The thermal stability of (III) up to 135 °C together with EPR evidence discount it as an intermediate in the formation of benzoxathiete or its tautomer cyclohexa-1,3-dien-5-one-6-thione.