ChemInform Abstract: Synthesis of Enol Esters and Enol Lactones via Palladium-Catalyzed Carbonylation of Aryl and Alkenyl Halides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
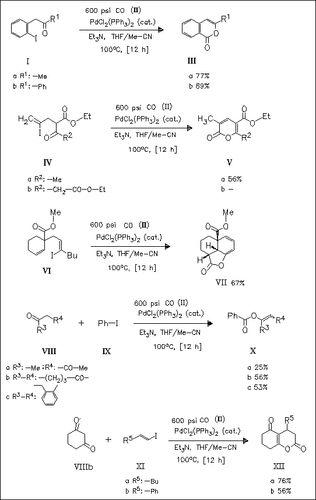
Palladium-catalyzed carbonylation of the γ-iodo ketones (I) and ( IV) forms the enol lactones (III) and (V). Carbonylation of the unsaturated iodo ester (VI) produces the tricyclic lactone (VII). Palladium-catalyzed reaction of the ketones (VIII) with iodobenzene ( IX) and carbon monoxide (II) gives the corresponding enol esters (X), whereas the enol lactones (XII) are obtained from (VIIb) and the vinylic iodides (XI) under analogous conditions.