ChemInform Abstract: Reduction of Substituted Nitro Compounds with Tri-n-butyltin Hydride.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
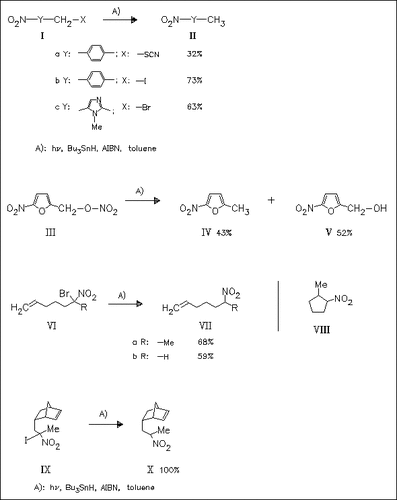
α-Substituted nitro compounds such as (I), (III), and (VI) are reduced by the title hydride to give the corresponding nitroalkanes. Applying low tributyltin hydride concentration the nitroalkane (VIIb) is accompanied by (VIII), a product of cyclization. No cyclic products are observed in the reduction of (IX), indicating that the bimolecular reduction of the intermediate α-nitroalkyl by Bu3SnH is faster than cyclization.