ChemInform Abstract: Synthesis and Biological Activity of 1,2,4-Oxadiazole Derivatives: Highly Potent and Efficacious Agonists for Cortical Muscarinic Receptors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
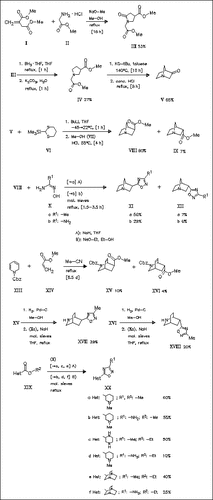
Dimethyl itaconate (I) reacts with methyl glycinate hydrochloride (II) to give the pyrrolidinone (III) which is reduced and subsequently cyclized, forming the 1-azabicyclo(2.2.1)heptan-3-one (V). This is coupled with 2-trimethylsilyl-1,3-dithiane (VI) and then methanolyzed to afford the esters (VIII) and (IX). Reaction of (VIII) with the amidoximes (X) yields the oxadiazoles (XI) and (XII). Diels-Alder reaction of the N-protected dihydropyridine (XIII) with methyl acrylate (XIV) produces the cycloadducts (XV) and (XVI) which are cyclized with (Xa) to form the oxadiazoles (XVII) and (XVIII). Analogously, the heterocyclic esters (XIX) are treated with (X) to give the oxadiazoles (XX). The antagonistic activity of the oxadiazoles prepared is studied, the derivative (XIb) is one of the most potent muscarinic antagonists known.