Tailoring Dynamic Surface Reconstruction on Nickel Oxalate for Enhanced Hydrogen Production and Zinc–Ethanol–Air Battery
ABSTRACT
Substituting the sluggish oxygen evolution reaction with a more thermodynamically favorable ethanol oxidation reaction (EOR) offers an opportunity to circumvent the efficiency loss in water splitting and metal-air batteries. However, the effect of the dynamic surface evolution of the catalyst in operating conditions on the activity of EOR lacks comprehensive understanding. Herein, we demonstrate a tunable operational catalyst activity through the modulated redox property of nickel oxalate (NCO) by establishing a relation between the oxidation behavior of Ni, surface reconstruction, and catalyst activity. We propose a repeated chemical–electrochemical reaction mechanism of EOR on NCO, which is rigorously investigated through a combination of operando Raman and nuclear magnetic resonance. The modulation of the oxidation trend of Ni by doping heteroatoms stimulates the electrochemical oxidation of the catalyst surface to NiOOH, which alters the catalyst activity for EOR. Assembled ethanol-assisted water electrolysis cell exhibits a reduced operating voltage for hydrogen production by 200 mV with a ~100% Faradaic efficiency, and zinc–ethanol–air battery showed a 287 mV decreased charge–discharge voltage window and enhanced stability for over 500 h.
1 Introduction
Hydrogen is an attractive energy carrier owing to its desirable characteristics, such as high energy density, industrial versatility, and environmental friendliness [1, 2]. In the face of the impending energy crisis, water electrolysis has emerged as a sustainable method for generating green hydrogen by converting water into hydrogen using intermittent electrical energy [3-7]. Despite extensive efforts to enhance hydrogen production efficiency, the anodic reaction of oxygen evolution suffers from slow kinetics and a high overpotential [8-10]. Additionally, the oxygen generated at the anode has minimal market value, and the separation of hydrogen and oxygen requires additional procedures and consumes significant energy [11]. To address these challenges, conceptual strategies have been recently developed aiming to replace the anodic oxygen evolution reaction (OER) with more favorable organic oxidation reactions [12, 13]. By coupling the hydrogen evolution reaction with the oxidation of various organic compounds, such as urea [14-18], methanol [19], ethanol [20, 21], glycerol [22], hydrazine [23, 24], and 5-hydroxymethylfurfural (HMF) [25], energy-efficient hydrogen production can be achieved by reducing the potential required for anodic reactions. Moreover, specific organic compounds, such as ethanol, glycerol, and HMF, offer an additional benefit of generating high-value organic products, contributing to biomass upgrading.
The OER also accounts for the anodic reaction during the charging process of metal–air batteries, hindering widespread commercialization [26, 27]. To substitute the anodic reaction with organic oxidation, a recent paper reported the incorporation of an organic compound into the electrolyte of a zinc–air–battery (ZAB) [28]. The voltage required for charging could be significantly reduced, indicating that the electric power consumption can be partly controlled by the direct utilization of the chemical energy of biomass fuels. Additionally, unlike in ZAB, where the oxygen gas bubbles from the OER can impair the catalyst, the oxidation of organics like ethanol produces liquid by-products, securing catalyst stability for semi-permanent operations. Consequently, replacing the OER with organic oxidation reactions is relevant not only for water electrolysis but also for energy storage applications such as metal–air batteries.
Among the diverse organic oxidation reactions available for hybrid applications, the ethanol oxidation reaction (EOR) has gained extensive attention owing to the low cost and wide availability of ethanol [28, 29]. Ethanol, which is easily produced through biomass fermentation, is nontoxic and can be easily stored and transported. Compared with the OER, the EOR is known to occur at a significantly lower potential of 0.084 V versus the reversible hydrogen electrode (RHE) under complete oxidation of ethanol [30]. Moreover, the oxidation of ethanol allows for the production of valuable chemicals, such as acetate and acetaldehyde [31]. Currently, most studies on the EOR have focused on noble-metal-based catalysts, such as Pt, Pd, and Rh, owing to their high catalytic activity and low reaction potential [32]. However, the high cost and scarcity of noble metals hinder large-scale commercialization, and they suffer from serious deactivation due to surface poisoning and oxidation during long-term operation [33]. Hence, the design and utilization of cheap, stable, and abundant transition metal-based catalysts are essential for the commercialization of EOR.
Recently, several attempts have been made to catalyze the EOR based on well-known 3d-transition metals, such as Ni, Co, and Fe [34, 35]. For 3d-transition metals, they offer a significant advantage by effectively redirecting the reaction pathway to minimize the formation of CO2 and produce valuable chemicals, such as acetate and acetaldehyde. To further enhance the catalyst performance of 3d-transition metal-based catalysts, various modification strategies, such as heteroatom doping [36, 37], vacancy engineering [34], heterostructuring [20, 38, 39], and nanostructuring [40, 41], had been actively investigated. Despite the apparent success in the correlations between bulk structure and the catalyst activity, there remains ambiguity surrounding the catalytic properties of transition metal-based catalysts under operating conditions due to the complex nature of the surface during anodic reactions. Studies revealed that most 3d-transition metal-based catalysts would undergo dynamic surface reconstruction to form transition metal-based oxyhydroxides, which often exhibit superior catalytic activity compared to their pristine counterpart [4, 42-45]. In particular, for Ni-based catalysts, the surface is reconstructed to nickel oxyhydroxide (NiOOH) in alkaline anodic conditions [46]. Reconstructed NiOOH, widely studied for its high catalytic activity toward the OER, also exhibits high electronic conductivity and considerable reactivity with organics, such as alcohols, facilitating anodic reactions [47-51]. Sun et al. employed NiOOH@CuO as a reactive species for the EOR and achieved superior performance toward hydrogen and acetate production [20]. Zhang et al. investigated active Ni-based chalcogenides for the EOR and achieved a low potential of 1.5 V at 70 mA cm−2 through in situ activation of NiSe2 into NiOOH under working conditions [52]. Despite various efforts to utilize these intriguing materials, there has been a lack of comprehensive understanding of the controllability of the actual catalyst activity under operating conditions. Furthermore, no previous work has demonstrated the ability to control the operational catalyst activity for organic oxidation reactions through a direct link between the occurrence of surface reconstruction and the EOR reaction.
In this study, we synthesized transition metal-doped nickel oxalate (NiC2O4, termed NCO) as an electrocatalyst for the EOR with rationally controlled surface reconstruction and catalytic activity. A facile room-temperature method was introduced for the synthesis of heteroatom-doped nanostructured NCO on nickel foam (NF). Co, Mn, and Fe were doped in NCO to manipulate the oxidation behavior of Ni2+, the surface reconstruction, and the catalyst activity of EOR. The equivalent potential values required for the three events established a rational approach for directly controlling the operational catalyst activity through the modulation of nickel redox properties. In situ observation of the dynamic surface reconstruction during EOR was performed via operando Raman spectroscopy to elucidate the reaction mechanism and confirm the surface phase tunability.
Among the synthesized catalysts, NiOOH@Co–NCO exhibited the highest level of performance with an ultralow potential of 1.340 V required to reach a current density of 100 mA cm−2, breaking the bottleneck of Ni-based catalysts. A hybrid water electrolysis cell was constructed to simultaneously realize hydrogen generation and acetate production with a high efficiency of 98%. Additionally, by replacing the cathode reaction of ZAB from OER to EOR, a zinc–ethanol–air battery (ZEB) was assembled, which showed a reduced charging voltage and high operational stability. This study provides a rational approach to tune the surface reconstruction and corresponding EOR activity for the development of high-performance electrocatalysts for energy-saving hydrogen production and innovative metal–air batteries.
2 Experimental Section
2.1 Preparation of Co-, Mn-, and Fe-Doped Nickel Oxalate
Before synthesis, NF was etched in 3.0 M HCl for 10 min to remove the surface oxide and then washed in deionized (DI) water and ethanol for 10 min each. The supported NCO was synthesized through a continuous precipitation–dissolution process where the NF was dipped in 1.0 M oxalic acid solution of ethanol and DI water (ethanol:DI = 19:1) for 90 min [53, 54]. Nickel is dissolved by the H+ ions present in the solution and then reacts with the oxalate anion (C2O4–) to form NCO species on the surface. This process was repeated to form a flake-like nanostructured NCO. After the chemical reaction, the foam was washed with DI water and ethanol and dried overnight at room temperature. Co, Mn, and Fe were doped onto the surface of NCO by dipping NCO each in 10 mM aqueous solutions of CoCl2 ∙ 2H2O, MnCl2 ∙ 4H2O, and FeCl3 ∙ 6H2O for 2 h [55, 56]. After the reaction, the foam was washed with DI water and dried at room temperature overnight. The obtained samples were denoted as Co–NCO, Mn–NCO, and Fe–NCO, respectively.
2.2 Characterization
The crystal structure of the synthesized catalysts was analyzed by X-ray diffraction (XRD, Rigaku Ultima IV) with Cu Kα radiation (λ = 1.5418 Å). The surface morphologies and microstructures were investigated using scanning electron microscopy (SEM, Hitachi SU8230). The nanostructure and surface crystallinity were examined using high-resolution transmission electron microscopy (HR-TEM) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM; Talos F200X). Energy dispersive spectroscopy (EDS) was performed using a Super-X 4 windowless SDD-EDS system. X-ray photoelectron spectroscopy (XPS) was employed to determine the surface chemistry using a Nexsa G2 instrument (Thermo Scientific). Raman spectroscopy was performed using an ARAMIS dispersive Raman spectrometer (Horiba Jobin Yvon) with a 514 nm light source and a long-working-distance 50× objective lens. Raman frequencies were calibrated using a Si wafer (520.6 cm−1) before each experiment.
2.3 Electrochemical Analysis
Electrochemical measurements for the EOR were performed on an electrochemical workstation (Bio-logic VMP-300) with a typical three-electrode cell employing an Ag/AgCl (3.5 M KCl) electrode as the reference electrode, a graphite rod as the counter electrode, and a mixture of 1.0 M KOH and 1.0 M ethanol as the electrolyte. The catalyst materials grown on the NF were directly used as the working electrodes.
All the potentials discussed in this work refer to the RHE, unless otherwise mentioned. Chronopotentiometry (CP) was performed to measure the long-term stability.
2.4 Acetate Production Analysis
2.5 Operando Raman Analysis
Operando Raman was conducted using a portable potentiostat (Bio-logic SP-200), and a three-electrode Raman cell was used for the measurements. The applied potential was maintained for 90 s before each measurement to ensure its equilibrium state. For the measurements, 8 mL of 1.0 M KOH and 1.0 M KOH + 1.0 M ethanol was used as the electrolyte. The acquisition time was set to 5 s, and complete spectra were acquired by averaging 10 scans. Only for measuring the spectral changes after ethanol addition, the data were averaged from five scans to reduce the time between each measurement.
2.6 Water Electrolyzer and Metal–Air Battery Evaluation
Ethanol-assisted and conventional water electrolysis cells were assembled with the synthesized NiOOH@Co–NCO as the anode and Pt/C/NF as the cathode. The ZAB and ZEB were set up with a 0.25 mm Zn plate as the negative electrode and Pt/C-coated NiOOH@Co–NCO as the positive electrode. We mixed 6.0 M KOH (for ZAB) and 6.0 M KOH + 1.0 M ethanol (for ZEB) solution with 0.2 M nickel acetate dihydrate and used the resulting solution as the electrolyte. The electrolyte was regularly replenished to supplement the ethanol fuel. Cycling charge–discharge tests were conducted at a current density of 5 mA cm−2 for 20 min per step.
3 Results and Discussion
3.1 Synthesis and Characterization of Transition Metal-Doped Nickel Oxalate
To construct large-scale commercial electrodes, a facile and scalable synthesis method is essential. In this study, the NF-supported transition metal-doped nickel oxalate (M–NCO) was prepared through a chemical dissolution–precipitation reaction and cation exchange method. Etched NF was dipped into an oxalic acid solution for the synthesis of nanostructured NCO, then dipped into an aqueous solution containing Co, Mn, and Fe cations for heteroatom doping. The entire process was performed at room temperature, without the need for any heat treatment or electrochemical processes. The morphology and nanostructure of the synthesized catalysts were investigated through SEM (Figure 1B–D) and HR-TEM (Figure 1E,F). The as-synthesized catalyst exhibited a nanoflake-like structure supported on an NF substrate. The length of each flake was approximately 300 nm, and the flakes were uniformly distributed throughout the foam. This unique 2D structure allows for a facile diffusion of the reactants and the product and enables robust mechanical stability during operation.
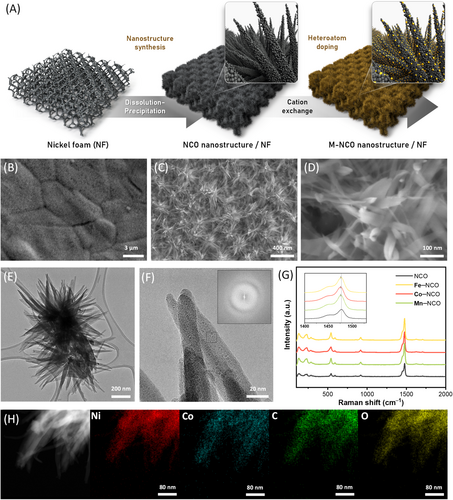
The nanostructure and crystallinity of the catalyst were further observed using HR-TEM. The synthesized NCO flakes had extremely low crystallinity mixed with amorphous phases, as shown in the TEM image and fast Fourier transform (FFT) pattern (Figure 1F). This is a well-known feature of nanostructures synthesized at room temperatures since low-temperature precipitation induces nanocrystalline or amorphous structures [58, 59]. The low crystallinity of the catalyst surface ensures considerable exposure of the active area and facile surface reconstruction during the reaction [60]. For the synthesis of Co-, Mn-, and Fe-doped NCO, the cation exchange method was employed. The morphologies of the as-synthesized catalysts were examined by SEM (Figure S1) and TEM (Figures S2 and S3), where the structure of doped NCO resembled that of pristine NCO, with no significant change in the nanostructure after the doping process. XRD was employed to confirm the formation and retention of the oxalate phase during the precipitation and cation-exchange process (Figure S4). The presence of the oxalate phase was also investigated through Raman spectroscopy (Figure 1G), where the shift of the ~1480 cm–1 peak of Co–, Mn–, and Fe–NCO suggests a change in Ni2+–O–Ni2+ vibration bending, which is attributed to the incorporation of alien cations (dopants) with different sizes and masses [61-64]. XPS was used to examine the surface chemistry and confirm the doping of transition metals. As shown in Figure S5, the XPS spectra of Co–NCO, Mn–NCO, and Fe–NCO exhibit the existence of the major nickel oxalate phase with designated characteristic peaks of Co, Mn, and Fe in each catalyst. EDS mapping was used for the elemental analysis of the Co-, Mn-, and Fe-doped NCO, where Co, Mn, and Fe were homogeneously distributed in each sample and accounted for 10.2%, 4.9%, and 4.8% of the total cation content, respectively. (Figures 1H, S2, and S3). The consistent signals of Co, Mn, and Fe in each catalyst, along with the above Raman and XPS results, confirmed the successful synthesis of homogeneously doped M–NCO using the proposed method. Lastly, the scalability of this method was confirmed by successfully synthesizing Co–NCO on a large-size NF. The morphology and catalyst performance of Co–NCO acquired from different parts of the large-size foam were evaluated through SEM and electrochemical tests, where the identical results demonstrated the reliable scalability of the proposed method (Figure S6).
3.2 Mechanism Analysis of EOR on Nickel Oxalate
To identify the active sites and reaction mechanism, operando Raman spectroscopy and NMR were employed with elaborate analysis procedures. The surface phase was observed in 1.0 M KOH electrolyte and in 1.0 M KOH electrolyte containing 1.0 M ethanol, by varying the applied potential on the catalyst. Raman peaks at 475 cm−1 (Eg) and 551 cm−1 (A1g) indicate the existence of the reconstructed NiOOH phase [65-67]. Different trends in the surface reconstruction behavior were observed in terms of the applied potential between the two electrolytes. As illustrated in Figure 2A, for the catalyst tested in pure KOH electrolyte, the formation of surface NiOOH can be immediately observed with the oxidation of Ni near 1.37 V, and its content rapidly increases with increasing potential. This indicates that substantial accumulation of the surface phase occurred as soon as Ni was oxidized. However, for the catalyst tested in KOH electrolytes containing ethanol, the intensity of the NiOOH peak increased steadily with the increase of applied potentials, indicating that surface NiOOH accumulation occurred at a lower rate compared to catalysts tested in pure KOH electrolytes. Moreover, the formation of an additional Ni(OH)2 phase was detected, as indicated by 437 and 494 cm−1 in the Raman spectra. Given that NiOOH is highly reactive to chemically oxidize alcohols, aldehydes, and amines, the formation of Ni(OH)2 implies the possible reduction of NiOOH during the EOR reaction [68]. Additionally, the reversibility of surface phases in terms of applied potential also implies the presence of an opposing reaction to NiOOH formation, which also supports the possibility of NiOOH consumption and Ni(OH)2 formation during EOR (Figure 2A). Based on the obtained results, a reaction mechanism for EOR on NCO was proposed to proceed through a combination of Ni electro-oxidation reaction for surface NiOOH formation, and a chemical reaction between NiOOH and ethanol (Figure 2B).
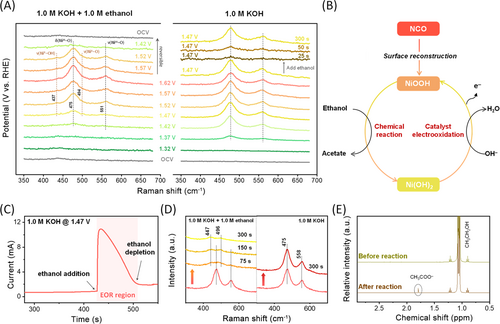
To further confirm the reaction mechanism, controlled in situ/ex situ experiments were conducted. First, the occurrence of EOR and surface phase transition within the addition of ethanol in a pure KOH electrolyte was observed. When ethanol was added while applying a constant potential of 1.47 V on the catalyst surface in the KOH electrolyte, a sudden decrease in the NiOOH peak was observed within a short time, indicating that the surface NiOOH was rapidly consumed by the reaction with ethanol. Chronoamperometry measurements were performed during this process to observe changes in the current where the decrease in the Raman signal for NiOOH was accompanied by a sudden surge in the current, signifying the occurrence of an oxidation reaction (Figure 2C). After a period of time, the current was recovered, indicating the consumption of the incorporated ethanol. This implies that the reaction between NiOOH and ethanol took part in the electrochemical ethanol oxidation reaction. To further verify the divisible electrochemical and chemical reactions, the reactions were performed sequentially in two separate systems. In the first cell, a potential of 1.47 V was applied to NCO to ensure the oxidation of Ni and the formation of surface NiOOH. The reconstructed NCO was then disconnected from the circuit and dipped in a KOH electrolyte and a KOH electrolyte mixed with ethanol. As shown in the Raman spectra of Figure 2D, the surface phase shifted from NiOOH to Ni(OH)2 when dipped in the ethanol-containing electrolyte, whereas no change was observed for the sample dipped in the pure KOH electrolyte. This further verifies that the electrochemical oxidation of Ni and the chemical reaction between NiOOH and ethanol can individually proceed, implying an indirect oxidation mechanism of the EOR, as shown in the schematic in Figure 2B. XPS analysis was also conducted to confirm the phase transformation of the NCO surface to nickel (oxy)hydroxide after EOR operation. (Figure S7) Finally, 1H NMR analysis of the electrolyte was performed before and after dipping the reconstructed NCO in an ethanol-containing cell to confirm the formation of acetate during the chemical reaction. For the pristine electrolyte, the peak at a chemical shift of 1.06 ppm was attributed to ethanol in the electrolyte (Figure 2E). After dipping, a peak at a chemical shift of 1.79 ppm was observed, indicating the formation of acetate from the oxidation of ethanol through a reaction between NiOOH and ethanol, in the absence of applied potentials.
With the oxidation of Ni, the NCO surface undergoes dynamic reconstruction to form NiOOH. Subsequently, the formed NiOOH rapidly reacts with ethanol to form acetate and is reduced to Ni(OH)2 (Equation 5). Finally, the formed Ni(OH)2 is electro-oxidized back to NiOOH through the applied anodic potential (Equation 6). In this section, the mechanism for EOR on NCO was elucidated through operando analysis. The key observation was that NiOOH forms rapidly upon Ni oxidation and then reacts with ethanol to produce acetate, reducing NiOOH back to Ni(OH)2. This phase transition between NiOOH and Ni(OH)2 during the reaction suggests a cyclic mechanism, where the chemical oxidation of ethanol by NiOOH and the electrochemical regeneration of NiOOH from Ni(OH)2 are crucial. These findings offer a deeper understanding of how surface dynamics and phase transitions influence the EOR reaction and highlight the significance of NiOOH regeneration in maintaining catalytic activity during the process. In the next section, the controllability of EOR activity through surface reconstruction modulation is demonstrated.
3.3 Modulation of Surface Reconstruction and Catalyst Activity
To characterize the surface reconstruction behavior of doped NCO, operando Raman spectroscopy was employed to identify the formation of the NiOOH phase on the catalyst surface (Figure 3). The potential for NiOOH formation was determined based on noticeable changes observed in the spectrum and was denoted as VR. The potential for Ni2+ oxidation was also analyzed through the LSV curves in a 1.0 M KOH electrolyte where the current density of the oxidation peak reached 2.0 mA cm−2 and is denoted as Voxi in the following description. As shown in Figure 3A, E, the potential for surface reconstruction (VR) of NCO was observed to be 1.37(2) V and Voxi was measured to be 1.36(2) V. The nearly identical values of Ni oxidation and surface reconstruction indicated that the surface was promptly reconstructed to form NiOOH immediately with the progression of Ni oxidation. This unique behavior of the catalyst is attributed to its low-crystalline structure, which makes it prone to dynamic reconstruction [60].
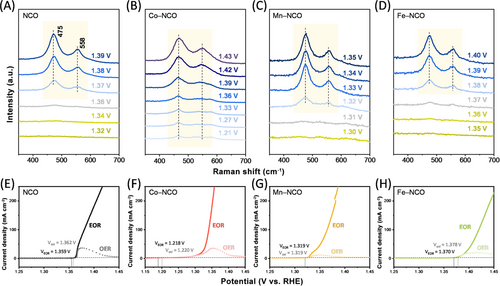
The doping of Ni-based compounds with transition metals, such as Co, Mn, Fe, Al, and Ti, is known to shift the oxidation potential of Ni2+ [28, 47, 67, 69]. We further investigated the controllability of nickel oxidation and corresponding NiOOH formation through surface reconstruction by doping NCO with Co, Mn, and Fe (Figure 3). For NCO doped with Co, Voxi and VR showed the lowest values of 1.220 and 1.21(2) V, respectively. For Mn–NCO, both VR and Voxi showed slightly increased values of 1.32(2) and 1.319 V, respectively. Fe–NCO showed the highest potential values for both VR and Voxi of 1.38(2) and 1.378 V, respectively. For all catalysts, the Ni oxidation potential and the potential for surface reconstruction exhibited nearly identical values, implying the rapid phase transformation with catalyst oxidation of both doped and undoped NCO.
The performance of EOR was also evaluated to demonstrate the controllability of catalyst activity. For Co- and Mn-doped NCO, the accelerated oxidation of nickel stimulated the formation of NiOOH, which led to enhanced catalyst activity toward the EOR compared to NCO (Figure 3F,G). In contrast, for Fe–NCO, the activity toward the EOR was reduced owing to the sluggish formation of NiOOH caused by the delayed oxidation of nickel (Figure 3H). The onset potentials of EOR for the synthesized catalysts were determined at a current density of 2.0 mA cm–2 where Co–NCO, Mn–NCO, NCO, and Fe–NCO each displayed an onset potential value of 1.21(8), 1.31(9), 1.35(9), and 1.37(0) V, respectively. The amount of surface reconstruction, which also could influence the catalyst activity, was investigated through electrochemical surface area analysis where all four catalysts exhibited similar degree of surface NiOOH formation (see Figure S8). Finally, the potentials for Ni oxidation, surface reconstruction, and EOR onset are illustrated in Figure S9 where all three potentials showed a near identical value for all catalysts. The clear alignment of nickel oxidation potential, surface reconstruction, and EOR onset across all catalysts shows that doping effectively controls catalytic activity by modifying nickel oxidation behavior. This finding underlines the importance of doping in fine-tuning the operational catalyst activity under reaction conditions.
3.4 Catalyst Performance of Surface Reconstructed Co-Doped Nickel Oxalate
The electrocatalytic performance of the synthesized catalysts is summarized in this section, where Co-, Mn-, and Fe-doped NCO after surface reconstruction are denoted as NiOOH@Co–NCO, NiOOH@Mn–NCO, and NiOOH@Fe–NCO, respectively. Surface reconstruction was induced on each catalyst through a single LSV measurement before activity evaluation. The LSV curves, shown in Figure 4A, depict the activity of each catalyst toward the EOR. Figure 4C shows the potentials required to reach a current density of 10 mA cm−2 for both the EOR and OER; NiOOH@Co–NCO exhibited the best EOR activity of 1.298 V at 10 mA cm−2, which was 222 mV lower than that of the OER. This value as well as the Tafel-slope (31.3 mV dec−1, Figure S10) is among the best levels achieved in 3d-transition metal-based catalysts for alkaline EOR (Figure 4B). The electrochemical double-layer capacitance (Cdl) was measured to confirm the electrochemically active surface area of the nanostructured catalyst before and after surface reconstruction (Figures 4D and S11). The measurements were conducted using cyclic voltammetry over a non-Faradaic potential region at various scan rates [70]. The Cdl values of NiOOH@NCO and NiOOH@Co–NCO were greater than those of the bare NF, indicating an increased surface area due to NCO precipitation on NF. The change in Cdl was more significant during surface reconstruction, implying an increased number of active sites after the formation of the surface NiOOH species. Additionally, Co−NCO with varying concentrations of Co dopants was synthesized to evaluate the impact of dopant concentration on the EOR activity of NCO and the oxidation behavior of Ni. By adjusting the concentration of the Co chloride solution used in the cation exchange process from 10 to 5 mM, the dopant concentration was modified from 10.2 to 3.2 at%. The STEM and EDS mapping results, shown in Figure S12, indicate that 3.2 at% of Co was evenly dispersed throughout the catalyst. The EOR activity was slightly reduced compared to Co−NCO (10.2 at% of Co), and the oxidation potential of Ni was slightly increased, highlighting the influence of dopant amount on EOR activity and Ni oxidation behavior. The durability of NiOOH@Co–NCO was evaluated by CP at a fixed current density of 100 mA cm−2 (Figure 4E). The electrolyte was regularly renewed to a 1.0 M KOH + 1.0 M ethanol solution during the long-term operation to accurately investigate the activity of the catalyst. NiOOH@Co–NCO exhibited a negligible potential increase during 100 h of operation, suggesting high catalyst stability. The morphology of the catalysts was also investigated after the stability test, where the nanostructure was maintained even after prolonged measurements, as shown in the SEM images (Figure S13). Moreover, no gaseous products were observed at the working electrode during the long-term analysis, implying the formation of only liquid products through the continuous oxidation of ethanol.
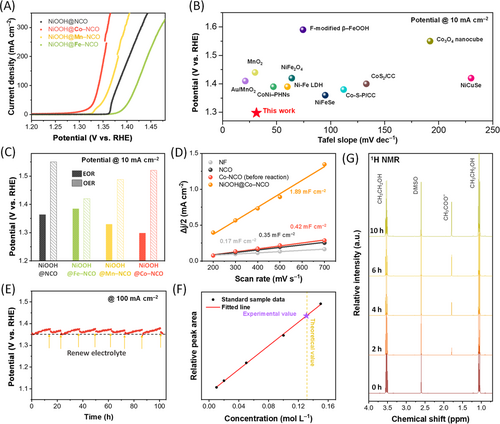
To quantitatively evaluate the production of acetate, 1H and 13C NMR were used. NiOOH@Co–NCO was used as the anode catalyst, and the reaction was performed at 100 mA cm−2 for 2, 4, 6, and 10 h. For the spectra obtained from the electrolyte before reaction, the peaks at 1.06 and 3.53 ppm are attributed to ethanol and the peak at 2.60 ppm corresponds to the DMSO, which was used as the internal reference. After the reaction, an additional peak at 1.79 ppm arose, indicating acetate to be the sole product of the EOR (Figure 4G), which was also confirmed through 13C NMR (Figure S14) [20, 47]. The FE of acetate production was evaluated by comparing the concentration of formed acetate with the theoretical value, where a high FE value of 98% was achieved after an operation of 10 h (Figure 4F) [28]. 1H NMR spectra of the reference samples used to construct the reference line of acetate are plotted in Figure S15. The production rate evaluated for various operation times indicated a stable formation of acetate for 2, 4, 6, and 10 h of operation with a maintained FE, successfully showcasing the exceptional durability of the synthesized catalyst (Figures 4G and S16).
3.5 Ethanol-Assisted Water Electrolysis and Zinc–Ethanol–Air Battery
Inspired by the intriguing performance of the catalyst, we constructed water electrolyzer and battery systems to further confirm the feasibility of hybrid applications. To demonstrate the performance of ethanol-assisted hybrid water electrolysis, a two-electrode electrolysis cell was assembled using NiOOH@Co–NCO as the anode and Pt/C/NF as the cathode. For the electrolyte, a mixed solution of 1.0 M KOH and 1.0 M ethanol was used. For comparison, a conventional hydrolysis cell was arranged with an identical configuration but with a 1.0 M KOH solution as the electrolyte. The conventional cell required a voltage of 1.67 V to reach a current density of 50 mA cm−2, where the voltage significantly reduced to 1.47 V with the addition of ethanol (Figure 5A). The voltages required to acquire current densities of 50, 100, and 150 mA cm−2 are compared in Figure 5B, where the ethanol-assisted water electrolyzer exhibited a voltage of nearly 200 mV lower than that of the conventional water electrolyzer for operation at various current densities. The FE was also evaluated at different current densities (Figure 5C) and operation times (Figure S17) to demonstrate the feasibility of ethanol-assisted water electrolysis. The cell exhibited a high FE of ~100% for hydrogen production at various operating current densities and reaction times where this value was maintained for current densities up to 400 mA cm−2, indicating stable operation under high current conditions. The durability of the catalyst was further investigated using CP at 100 mA cm−2 where no notable decrease in the activity was observed during 30 h of operation, implying high stability of the assembled electrolyzer (Figure S18).

By replacing the OER with the EOR, we demonstrated a ZEB system, as shown in Figure 5D. In the conventional ZAB, OER occurs at the air electrode and zinc precipitation occurs at the counter electrode during the charging process. With the addition of ethanol in the electrolyte, the cathode reaction can be shifted from the OER to the EOR, which effectively reduces the potential required for the charging process. This is shown in the polarization curves of ZEB and ZAB in Figure 5E, where the ZEB exhibited a 257 mV decreased charging voltage compared to the ZAB. In conventional ZAB, oxygen gas bubbles produced during the charging process damage the electrode and lead to deactivation [71]. However, for ZEB, this effect is alleviated since the EOR reaction only produces liquid-phase products. This can be seen by the distinct cycling stabilities of ZAB and ZEB, as illustrated in Figure 5F. Conventional ZAB showed a decaying activity during continuous operation and deactivation after 330 h. However, with the addition of 1.0 M ethanol, the durability could be significantly improved to achieve stable operation for more than 500 h. The morphology of the catalyst was examined after operation in both the ZAB and ZEB using SEM analysis, as shown in Figure S19. The preserved morphology of the catalyst following ZEB operation, in contrast to the catalyst after ZAB operation, further supports the enhanced stability of the ZEB. The charge–discharge window was also decreased from 897 to 609 mV, indicating the feasibility of shifting the cathode reaction from the OER to the EOR for battery applications. Our research, serving as the second paper to empirically validate the innovative concept, carries great significance by reaffirming this novel idea with the demonstration of stable operation for an elongated time by nearly 5 times that of the previous report.
4 Conclusions
In this work, we successfully demonstrated the controllable catalyst activity of EOR through modulation of surface reconstruction by introducing heteroatoms in nickel oxalate. The parallel behavior of nickel oxidation, surface reconstruction, and EOR activity was revealed for the first time through a mechanistic analysis, which settled a rational approach to tuning the catalyst activity of organic oxidation reactions. Co-doped nickel oxalate exhibited an exceptional EOR performance of 1.340 V to reach a current density of 100 mA cm−2 with high operational stability. By replacing the OER with the EOR, the hybrid water electrolysis cell displayed a reduced cell voltage of 200 mV for hydrogen production, and the ZEB showed a reduced charge–discharge window of 257 mV with exceptional stability for over 500 h. We believe that our systematic investigation into the correlation between in situ surface reconstruction and catalytic activity, along with innovative demonstrations, can serve as theoretical and practical guidance, thereby charting a course toward the synthesis of electrocatalysts for hydrogen production and pioneering applications.
Author Contributions
Yong Beom Kim: conceptualization, methodology, writing–original draft. Sangwoo Kim: validation, methodology. Yeongtaek Hong: formal analysis. Jeongah Lee: methodology, investigation. Hainan Sun: supervision, writing–review and editing, project administration. WooChul Jung: supervision, writing–review and editing, project administration, funding acquisition.
5 Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (NRF-2022M3H4A1A04076616 and NRF-2022M3H4A1A01008918) and has been performed as a cooperation project of “Basic project (referring to projects performed with the budget directly contributed by the Government to achieve the purposes of establishment of Government–funded research Institutes)” and supported by the KOREA RESEARCH INSTITUE of CHEMICAL THECNOLOGY (KRICT).
Conflicts of Interest
The authors declare no conflicts of interest.




