Insights on advanced g-C3N4 in energy storage: Applications, challenges, and future
Abstract
Graphitic carbon nitride (g-C3N4) is a highly recognized two-dimensional semiconductor material known for its exceptional chemical and physical stability, environmental friendliness, and pollution-free advantages. These remarkable properties have sparked extensive research in the field of energy storage. This review paper presents the latest advances in the utilization of g-C3N4 in various energy storage technologies, including lithium-ion batteries, lithium-sulfur batteries, sodium-ion batteries, potassium-ion batteries, and supercapacitors. One of the key strengths of g-C3N4 lies in its simple preparation process along with the ease of optimizing its material structure. It possesses abundant amino and Lewis basic groups, as well as a high density of nitrogen, enabling efficient charge transfer and electrolyte solution penetration. Moreover, the graphite-like layered structure and the presence of large π bonds in g-C3N4 contribute to its versatility in preparing multifunctional materials with different dimensions, element and group doping, and conjugated systems. These characteristics open up possibilities for expanding its application in energy storage devices. This article comprehensively reviews the research progress on g-C3N4 in energy storage and highlights its potential for future applications in this field. By exploring the advantages and unique features of g-C3N4, this paper provides valuable insights into harnessing the full potential of this material for energy storage applications.
1 INTRODUCTION
The energy crisis and environmental pollution have become important factors restricting human progress.1-3 At present, fossil fuels still dominate energy consumption, and the increasing demand for energy and the depletion of fossil fuels have led to a continuous deterioration of the supply–demand balance.4-6 The problems of atmospheric pollution, acid rain, climate change, glacier melting, and the greenhouse effect caused by fossil energy consumption have seriously affected human normal life.7-10 Therefore, it is urgent to develop clean and renewable secondary energy represented by solar and wind energy. Due to the uneven geographical distribution and unstable output of clean energy sources, however, the energy utilization rate of renewable energy is very low, resulting in resource waste. Therefore, it is urgent to develop large-scale energy storage systems and store clean energy in other forms.11, 12
Lithium-ion batteries (LIBs), sodium-ion batteries (SIBs), and other representative secondary batteries are widely used in electrochemical energy storage systems.13-20 They have become an important means to solve energy, resource, and environmental problems due to their recyclability and environmental friendliness.21, 22 Secondary batteries, which have the advantages of high energy density, short response time, and good flexibility, are electrochemical energy storage/release devices that utilize redox reactions to achieve reversible conversion between electrical and chemical energy.23-28 Their working principle lies in the reversible deinsertion of electrolyte ions between positive and negative electrode materials to achieve energy storage. According to the different electrolyte ion/electrode materials, the secondary batteries can be divided into LIBs, lithium-sulfur batteries (LSBs), SIBs, potassium-ion batteries (PIBs), and so forth. Figure 1 is a schematic illustration of the working principle of energy storage devices.29-32
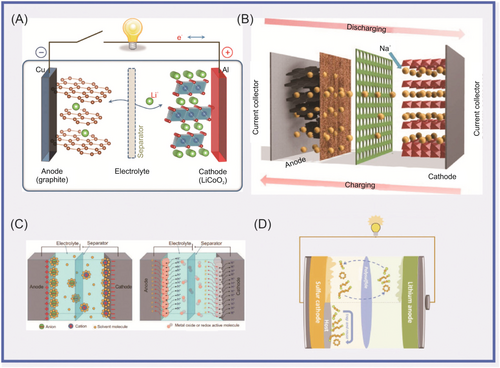
1.1 A brief introduction to graphitic carbon nitride (g-C3N4)
Battery materials encompass various components, notably electrodes, separators, and more.33, 34 They serve as essential elements in energy storage devices. For researchers, there is a growing interest in investigating batteries with high specific capacity, superior energy density (both bulk and quality), elevated power density, prolonged cycle life, and impeccable safety standards, leading to challenging yet promising research avenues.35-40 Among these materials, electrodes and diaphragms emerge as pivotal focuses for energy storage device advancements.41-43 Specifically, electrode materials are categorized into positive and negative types, each presenting its unique merits and drawbacks in the context of energy storage.44, 45 The primary issues concerning positive electrode materials include limited specific capacity, phase transition during charge–discharge cycles, cycle life, and interface reactions.46-48 These challenges call for dedicated research and development efforts to overcome these limitations and unlock the full potential of energy storage technologies. The primary challenges associated with anode materials encompass electrode material agglomeration, specific capacity, safety concerns, phase and volume changes during charge–discharge cycles, cycle life, and rate performance.49, 50 Researchers are actively exploring various strategies to address these issues, such as nanostructure design, lattice doping, grain boundaries, surface treatment, particle size reduction, carbon coating, element doping, and the development of composite materials.51-55 Furthermore, the separator plays a vital role in a battery, not only ensuring its fundamental functionalities but also significantly impacting its overall safety. Careful attention and research are devoted to enhancing the separator's properties to guarantee the reliable and secure operation of batteries.56-58 Simultaneously, the diaphragm serves as the carrier of the electrolyte, playing a pivotal role as it becomes infused with the electrolyte, thereby establishing an essential pathway for ion transport. Consequently, the diaphragm's characteristics significantly influence the electrolyte's wettability, pore structure, and overall electrochemical performance of the energy storage device. Researchers acknowledge the critical impact of the diaphragm's properties on optimizing the device's efficiency and functionality, leading to extensive investigations in this domain.59-63
There are four main kinds of synthesis methods for the preparation of g-C3N4, including thermal polymerization method, solvothermal method, template-assisted method, and photopolymerization method. Among these, g-C3N4 is easily fabricated by thermal polymerization of abundant nitrogen-rich precursors such as melamine,64-69 dicyandiamide,70-73 cyanamide,74, 75 urea,76, 77 thiourea,78 and ammonium thiocyanate.79 g-C3N4 exhibits two fundamental structural units: the triazine ring (C3N4) and the tris-triazine ring (C6N7).65, 80 The triazine ring comprises N atoms outside the ring connected to a single C3N4 unit, while the tris-triazine ring consists of N atoms outside the ring linked with three polymerized C6N7 units.77, 81-84 These structural units, composed of arranged C and N atoms, form a gap-like configuration, and each unit extends outward, connecting with N atoms as nodes, resulting in a planar layered structure with limitless expansion possibilities. To enhance the applicability of g-C3N4 in energy storage devices, incorporating functional groups into its molecular structure is advantageous. The introduction of such functional groups can improve the material's performance and properties, making it more suitable for energy storage applications.85-87 Researchers are exploring this avenue to design g-C3N4 molecules with tailored functional groups to optimize their performance in energy storage devices. This development holds great promise for advancing energy storage technologies.
The discovery of g-C3N4 as a metal-free conjugated semiconductor photocatalyst for H2 evolution was first reported by Wang et al. in 2009,88 which may also lead to research and exploration of the potential for application of inorganic conjugated semiconductor materials in energy storage.89-91 It was found that the basic tectonic units that establish allotropes of g-C3N4 are triazine (C3N3) and tri-s-triazine/heptazine (C6N7) rings.92, 93
Since polymeric g-C3N4 consists of earth-abundant carbon and nitrogen elements, it is versatile in providing reactions to alter its surface activity without manifestly changing its theoretical structure and composition. Due to the polymeric feature of g-C3N4, its surface chemistry can be easily modulated by means of surface engineering at the molecular level. As shown in Figure 2, the surface of g-C3N4 is rich in amino groups (–NH2) and nitrogen, which is conducive to its functionalization.94, 95 Furthermore, it is the most stable among the five phases (α, β, cubic, pseudocubic, and graphitic) of C3N4, and establishes the π-conjugated electronic structures owing to the presence of sp2-hybridized carbon and nitrogen.96 g-C3N4 has a moderate band gap of 2.7–2.8 eV, resulting in the onset of visible light absorption at around 450–460 nm.81 g-C3N4 is thermally stable up to 600°C in air, which can be ascribed to the aromatic C–N heterocycles. g-C3N4 is a metal-free, earth-abundant, and nontoxic material with a polymeric two-dimensional (2D) structure.97 It has been widely used in pollutant degradation, water splitting, and solar energy transfer due to its outstanding photocatalytic and optoelectronic properties.91, 98-100 The lone pair of its nitrogen atoms and electron delocalization endows the tri-s-triazine derivatives, including g-C3N4, with a unique electronic structure,101-103 which was controllable due to their tunable band gap. Furthermore, the excellent thermal and chemical stability of g-C3N4 make it one of the most promising semiconductor materials in this exciting research field.
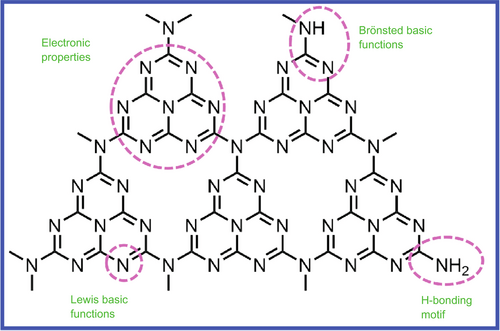
2D g-C3N4 materials possess outstanding physical and chemical properties, rendering them highly versatile in various applications. One significant advantage lies in their capability to act as carriers for loading diverse nanomaterials. This property enables them to serve as excellent platforms for hosting and supporting different nanoparticles (NPs), thus facilitating the development of advanced composite materials with enhanced functionalities. Additionally, g-C3N4 materials exhibit the ability to encapsulate and protect other materials. By acting as protective shields, they safeguard sensitive components from external factors, such as harsh environments or chemical reactions, thereby preserving the integrity and performance of the encapsulated materials. These remarkable attributes open up promising avenues for their utilization in numerous fields. As carriers and protective agents, 2D g-C3N4 materials hold great application prospects and significant value for further advancements in various industries and research areas.
1.2 Scope of this review
To date, numerous excellent review papers have focused on key materials for rechargeable batteries, emphasizing safety performance, energy density, and battery cost. To the best of our knowledge, however, there is a dearth of articles discussing multifunctional materials. This significant gap in the literature has motivated us to thoroughly review and elucidate the recent progress achieved by using g-C3N4 for the fabrication of high-performance rechargeable batteries and supercapacitors. In this comprehensive review, we primarily focus on the application of g-C3N4 as a multifunctional material in energy storage devices. Additionally, we explore potential future developments and research directions in the field of energy storage devices. With its unique atomic structure, electronic configuration, and chemical stability as a triazine framework material, g-C3N4 has found widespread use in energy storage applications. Its distinctive electronic structure facilitates structural regulation and the construction of conjugated systems, enabling multifunctionality and expanding its application in electrode materials and electrolytes for energy storage devices. This review serves as a comprehensive guide, shedding light on the promising advances and future prospects of utilizing g-C3N4 in energy storage devices. By examining the distinctive features and capabilities of g-C3N4, this paper not only provides valuable insights but also paves the way for further exploration and innovation in the realm of multifunctional materials for energy storage.
2 THE ROLES OF G-C3N4 IN ENERGY STORAGE DEVICES
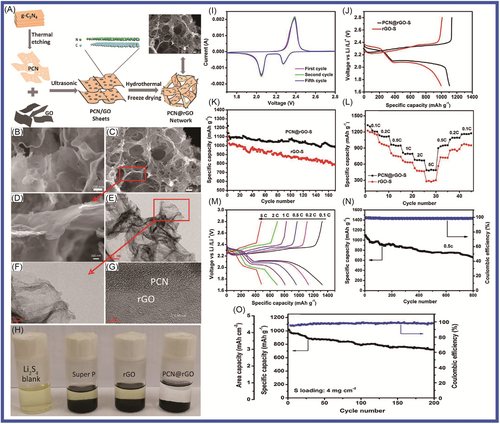
g-C3N4 has been the most widely reported member of the carbon nitride family, as its macropores serve as storage sites for alkali metal atoms. The adsorption of Li atoms at these triangular pore sites occurs at a high adsorption energy (Ead) of ~4.2 eV, exceeding the desorption energy (3 eV) of bulk Li, resulting in ineffective desorption. The modulation of vacancies has been an attractive strategy to improve the conductivities of semiconductor materials for high-performance electrodes in LIBs. g-C3N4 can be regarded as a special nitrogen-doped carbon material (C-NC), which has nitrogen-rich properties and good physical and chemical stability. It has great potential applications in electrochemical storage (SIBs, LIBs, LSBs, supercapacitors, etc.) and other fields in Figure 3. However, there are several challenges that researchers and engineers need to address for its successful application in these devices, including low conductivity, limited charge storage structural stability, scalability production cost, and electrolyte compatibility, which limits its application in electrochemistry. To improve the level of application, it is necessary to optimize and modify the material.
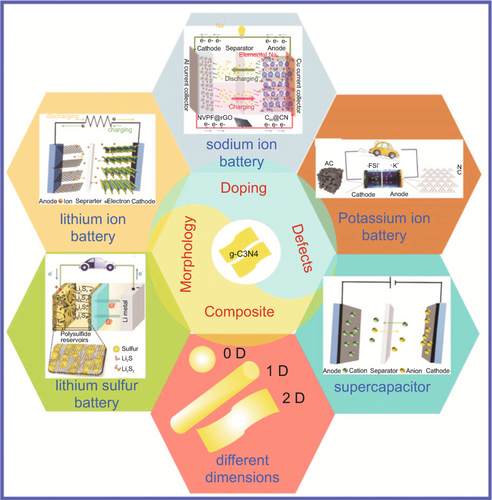
2.1 g-C3N4 applied in LIBs
With the deepening of environmental pollution and the energy crisis, it is imperative to develop clean, efficient, and sustainable electrochemical energy storage devices.104, 105, 107-109 When graphite was used as the negative electrode material for commercial LIBs, it had a theoretical low specific capacity (372 mAh g−1) and slow lithium-ion diffusion, which made it unable to meet the capacity requirements of rapidly upgraded portable electronic devices and electric vehicles.110, 111 g-C3N4 as a negative electrode material for LIBs has a high theoretical specific capacity (1165.7 mAh g−1) and high stability, exhibiting high conductivity and low reversible capacity.112-114 The optimization modification of g-C3N4 could overcome the shortcomings of low conductivity and irreversible capacity of the material by removing some graphite N from the structure.115, 116
Chen and colleagues117 reported that N-doped graphene/porous g-C3N4 nanosheets supporting layered-MoS2 hybrid, which were applied in LIB anode, had been designed and prepared. To form a good 2D multilayered nanostructure, few-layer MoS2 nanosheets were highly dispersed on the N-doped graphene/porous g-C3N4 nanosheet matrix. Benefiting from this unique structure, the hybrid nanosheets exhibited superior electrochemical performance, which included excellent cycling stability (maintaining 91% capacity after 100 cycles), a high rate capability (retaining 83% capacity from 50 to 500 mA g−1), and a large capacity (achieving more than 800 mAh g−1 at 100 mA g−1). Wang and colleagues118 designed and prepared g-C3N4-rGO, where rGO is reduced graphene oxide via an in situ chemical synthetic approach, which considered differing g-C3N4/rGO ratios in LIBs. The g-C3N4-rGO exhibited stable and reversible capacity (1525 mAh g−1 at current density of 100 mA g−1 after 50 cycles). It delivered high performance (943 mAh g−1 at the large current density of 1000 mA g−1). The high electrochemical performance of g-C3N4-rGO was attributed to the specific characteristics of its unique nanostructure (covalent interactions between the two moieties, good conductivity, and high special surface area of the nanocomposite [NC]) of g-C3N4-rGO. Li et al.119 reported that Zn2GeO4 NPs and ultrathin g-C3N4 layers (Zn2GeO4/g-C3N4 hybrids) were synthetized by a facile solution approach. Zn2GeO4/g-C3N4 served as anode in LIBs, which delivered high performance (1370 mAh g−1 at 200 mA g−1 after 140 cycles and excellent rate capability of 950 mAh g−1 at 2000 mA g−1). This excellent performance was mainly attributable to Zn2GeO4 and g-C3N4, in which the Zn2GeO4 NPs contributed high capacity. The Zn2GeO4 NPs were loaded on g-C3N4 layers via in situ growth, in which the g-C3N4 layers acted as an effective substrate for nucleation. To improve the electrochemical performance of graphene-based materials in LIBs, a novel composite of active Fe2O3 NPs encapsulated in g-C3N4/graphene hybrid nanosheets (Fe2O3/CN-G) was developed by Gao and colleagues.120 The Fe2O3/CN-G was applied in LIBs as an anode, which showed excellent electrochemical behavior (large reversible capacity of 1023 mAh g−1, great Coulombic efficiency of 97.6%). The 2D sandwich-type hybrid nanosheets were constructed from porous g-C3N4 and highly conductive graphene, which offered readily accessible channels and sufficient conductive pathways for ionic diffusion and charge transport due to the shortcomings of pure g-C3N4 material, in which poor conductivity and serious irreversible capacity loss were pronounced, owing to its high nitrogen content. Mao and colleagues121 prepared nitrogen-deficient g-C3N4 (ND-g-C3N4), which exhibited a thinner and more porous structure composed of an abundance of relatively low-nitrogen-doped wrinkled graphene nanosheets and showed enhanced lithium storage properties as LIB anodes (storage capacity of 2753 mAh g−1 after 300 cycles), via magnesiothermic denitriding technology. Mao and colleagues122 fabricated highly N-doped graphene-like carbon (NGC) through the carbonization of a mixture containing polypyrrole (PPy) and g-C3N4 nanosheets. The NGC material, which integrated a range of excellent features, including high nitrogen content (17.54 at%), large specific surface area (635.73 m2 g−1), and abundant mesopores with high pore volume (1.9483 cm3 g−1), was used as anode material in LIBs. The NGC material exhibited a promising initial discharge capacity of 2749 mAh g−1 at current density of 50 mA g−1. It had a high specific capacity of 1143 mAh g−1 at current density of 50 mA g−1 after 200 cycles and excellent rate performance with 440 mAh g−1 at 2 A g−1. Chenrayan et al.123 reported the construction of N-rich C3N4/MoS2 nanosphere from 2D layered materials, which served as a potential anode material for LIBs, delivering a reversible capacity of 857 mAh g−1 at 0.1 C rate for 50 cycles in Figure 4. It has superior rate performance of 383 mAh g−1 at 10 C rate. The excellent performance was mainly attributed to its excellent structure through the rapid electron transfer of nitrogen-rich g-C3N4 and the large electrode/electrolyte contact area of MoS2. g-C3N4/MoS2, in which the nitrogen-rich carbon and interconnection properties of the g-C3N4/MoS2 structure contributed to good electronic conductivity and a shorter diffusion length and provided mechanical flexibility to adapt to volume changes and the formation of heterostructures. Sasidharan and colleagues124 reported anatase TiO2 nanosphere (TNS) wrapped in nitrogen-rich carbon nanosheets (TiO2@CNSs, TCNSs), which were a robust and facile self-assembly approach involving titanium alkoxide and CNS under hydrothermal conditions followed by heat treatment. The TCNS nanostructures (mass loading of TiO2/CNS = 1.2) displayed very high lithium-ion storage capacity, 303 mAh g−1 at 0.1 C after 125 cycles and 136 mAh g−1 at 5 C after 500 cycles with high Coulombic efficiency (99%). The excellent performance was mainly attributed to the synergistic effect promoting structural flexibility and mechanical stability, as well as the electronic and ionic conductivity of TCNS composites, which provided excellent lithium storage, cycle life, and rate performance.
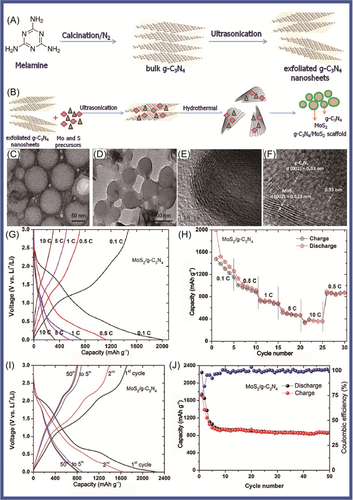
Vo et al.125 reported the preparation of an SnO2 nanosheet/g-C3N4 (SN/CN) composite, in which SnO2 nanosheets were agglomerated to form a cauliflower-like morphology with the assistance of mercaptoacetic acid in a hydrochloric acid medium. The SN/CN composite exhibited improved cycling performance, which was attributed to the presence of g-C3N4, compared to the stand-alone SnO2 nanosheets. Subsequently, Vo and colleagues126 reported that an SN/GO/g-C3N4 (SGC) composite had been prepared via the hydrothermal method, in which the SnO2 nanosheets were grown on GO/g-C3N4 support. In the first cycle, the discharge capacity of 1230.6 mAh g−1 at 0.1 C and the initial Coulombic efficiency of the SGC were obtained. The GO, which provided an electron- and ion-conductive medium, leading to good reaction kinetics, was introduced into SnO2 nanosheets/g-C3N4. The mesoporous g-C3N4 nanosheets were first introduced into the GO to form composite materials, which could support the formation of a stable solid-electrolyte interphase (SEI) layer, accommodating the volume changes during the lithiation/delithiation process and significantly improving the cycling performance of the electrode. To improve device performance, Vo and colleagues127 synthetized WS2/g-C3N4 composites via a facile one-step solid-state reaction. Their WS/CN-5 sample delivered a reversible capacity of 622.7 mAh g−1 after 400 cycles with a high current density of 1000 mA g−1, indicating excellent lithium storage performance.
2D nanomaterials, which have high gravimetric capacity and rate capability, were a key strategy for the LIB anode, although they still posed a challenge for lithium-ion storage. Their limited conductivity and inability to alleviate the volume changes upon lithiation and delithiation limited their application in LIBs. Goodenough and colleagues128 prepared an anode with three-dimensional (3D) structure via the hydrothermal method, which consisted of layers of 2D layered g-C3N4 and rGO nanosheets (CN rGO). It exhibited a capacity of 970 mA h g−1 after 300 cycles, which was 15-fold higher than for the BCN. Yin et al.129 synthesized SnS2/g-C3N4 (SnS2/CN) nanosheets by a facile microwave hydrothermal method, which consisted of hierarchical nanoflower SnS2 anchored on g-C3N4 nanosheets. This sample delivered a high discharge capacity of 444.7 mAh g−1 (at current density of 100 mA g−1 after 100 cycles) with a Coulombic efficiency of over 99.9%. It had a high specific capacity of 383.8 and 350.8 mAh g−1 at current density of 800 and 1000 mA g−1, respectively. The performance of the SnS2/CN composite, which provided more active sites and accelerated the migration of electrons and lithium ions during charge/discharge cycles, was attributed to its large surface area. To solve the agglomeration problem of graphene in LIBs and improve its performance, Sun et al.130 prepared a layered g-C3N4@rGO composite (g-C3N4@rGO) via a scalable and easy strategy, as shown in Figure 5. This material showed excellent performance (cycling stability of 899.3 mAh g−1 after 350 cycles under 500 mA g−1 and remarkable rate performance of 595.1 mAh g−1 after 1000 cycles under 1000 mA g−1), which was mainly attributable to its large interlayer distances, rich N-active sites, and microporous structure. Interestingly, the g-C3N4@rGO-based electrode was enough to power two tandem red-light-emitting diodes and run a digital watch, which was able to work continuously for more than 20 days. Wang and colleagues131 reported that a composite with cubic Co3O4 nanocrystals anchored on chemically integrated g-C3N4-modified N-graphene (CN-NG), which was applied in LIBs, had been synthesized via a facile strategy. It delivered a high specific reversible capacity (capacity of 1096 mAh g−1 at current density of 100 mA g−1) with excellent cycling stability and rate capability. The Co3O4/CN-NG composite material provided paths for the insertion/extraction of Li+ and buffered volume changes during charging and discharging. Sasidharan and colleagues132 synthesized g-C3N4@rGO hybrid materials, in which 2D layered g-C3N4 and GO were formed by self-assembly via a single-step hydrothermal reaction. The g-C3N4@rGO hybrid material as an anode material for LIBs exhibited excellent electrochemical performance (capacity of 901 mAh g−1 at the 0.1 C rate and 302 mAh g−1 at the 5 C rate). Tang and colleagues133 reported that g-C3N4/MoS2/ZnS composites, in which MoS2/ZnS heterojunctions were tightly anchored onto the surfaces of the g-C3N4 nanosheets, had been synthesized by a one-step hydrothermal method. The g-C3N4/MoS2/ZnS ternary NCs exhibited high capacity and excellent cycling stability, 1310 and 805 mAh g−1 at current densities of 0.1 and 1 A g−1, respectively.
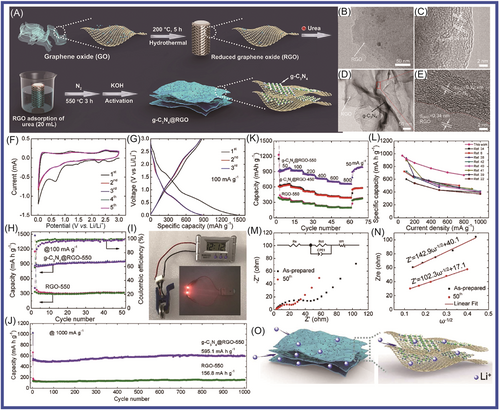
To improve the electrochemical performance of MnO-based anode materials, which were applied in high energy-density LIBs, Chen and colleagues134 reported that porous MnO/g-C3N4/carbon composite spheres had been synthesized through an aerosol–pyrolysis route. The porous MnO/g-C3N4/carbon composite spheres (with g-C3N4/carbon content of 8.6 wt%) displayed excellent electrochemical properties, with the first-cycle discharge capacity reaching 1096.8 mAh g−1 at 0.2 C. The discharge/charge capacities reached 918.9/605.8 mAh g−1 at 0.5 C. The highest reversible capacity was 781.9 mAh g−1, which was higher than the theoretical capacity of MnO (755 mAh g−1). This material showed good stability, and the reversible capacity retention reached 99% after 150 cycles at 0.5 C. Nitrogen atom doping has been widely recognized as a promising technique to improve the electrochemical performances of various carbonaceous materials applications for energy storage devices. NiCo2O4, which showed high performance due to its better electrochemical activity and higher capacity compared to traditional simple oxides, was regarded as a desirable electrode material in LIBs. Shao and colleagues135 coupled nanoparticulate NiCo2O4 with g-C3N4, which effectively reduced SEI formation and led to a high initial Coulombic efficiency. The NiCo2O4/g-C3N4 hybrid materials had excellent performance, with a capacity of 1252 and 476 mAh g−1 after 100 cycles at current density of 100 and 500 mA g−1, respectively. The Li4Ti5O12/g-C3N4 composite, in which g-C3N4 acted as a support to enhance the electronic conductivity of Li4Ti5O12, was prepared by the solvothermal method followed by heat treatment at 700°C for 5 h for the first time by Sun and colleagues.136 When the Li4Ti5O12/g-C3N4 was applied in LIBs as anode material, the Li4Ti5O12/g-C3N4 displayed a reversible capacity of 150.8 mAh g−1 with a capacity retention of 86.8% at 0.5 C after 502 cycles. Its excellent electrochemical performance was mainly attributed to the existence of the g-C3N4 support. As shown in Figure 6, Su and colleagues137 reported that copper oxide (CuO)/O-doped g-C3N4 nanospheres had been prepared by a two-step hydrothermal process at 180°C followed by annealing in air at 300°C. When the CuO/O-doped g-C3N4 was used as anode material, it exhibited high performance (specific capacity of 738 mAh g−1 at current density of 100 mA g−1 and specific capacity of 503 mAh g−1 at current density of 1 A g−1). The CuO/O-doped g-C3N4 nanospheres showed excellent performance, which was mainly attributed to the fact that O-doped g-C3N4 could effectively avoid agglomeration of CuO NPs during in situ growth, which is conducive to lithium-ion transfer and electrolyte penetration; the buffer volume of the porous structure accommodated the changes during the insertion/removal of Li+; and the O-doped g-C3N4 exhibited a reduced band gap and improved electrical conductivity. Mullins and colleagues138 synthesized C3N4-Cu and C3N4-Fe nitrogen-doped carbon. Compared with alloying Fe (carbon with a high degree of graphitization, low nitrogen content (<10%), and medium pore volume), the carbon formed by nonalloying Cu has high defects with high nitrogen content (32%–24%) and large pore volume. The C3N4-Cu (since Cu templating is beneficial to Li+ storage, with the carbon formed at 750°C) exhibited the highest specific capacity of 900 mAh g−1 at 0.1 A g−1 and 275 mAh g−1 at 20 A g−1. It retained 96% of its initial capacity after 2000 cycles at 2 A g−1. To improve the electrochemical performance of LIBs, composite electrode materials were designed and manufactured. Hierarchical nanoflower SnS2 anchored on g-C3N4 nanosheets (the SnS2/CN composite) was synthesized via a facile microwave hydrothermal method by Yin et al.139 The SnS2/CN composite material delivered excellent performance (cycling stability, high rate capacity, and structural integrity) at higher current density for LIBs. It exhibited a specific capacity of 444.7 mAh g−1 with a current density of 100 mA g−1 after 100 cycles with a Coulombic efficiency of over 99.9%. It exhibited a specific capacity of 383.8 and 350.8 mAh g−1 at current densities of 800 and 1000 mA g−1, respectively. The SnS2/CN composite materials, which provided more active sites and accelerated the migration of electrons and lithium ions during charge/discharge cycles, showed remarkable electrochemical performance.
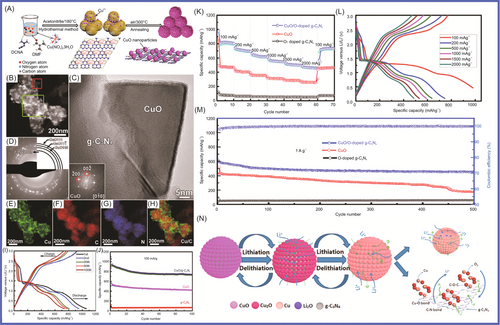
Nanostructure provided a way to solve the volume change for LIBs during charging and discharging. Fu and colleagues140 fabricated nanosized spinel ferrites, MFe2O4 (M = Mn, Ni, Cu, and Co) and g-C3N4 covalently functionalized nitrogen-doped graphene (CN-NG), which were formed in a 3D heteroarchitecture via a general approach, combining a self-assembly process, in situ substitution, and thermal annealing. The 3D MnFe2O4/CN-NG (0.4), 3D CuFe2O4/CN-NG (0.4), 3D CoFe2O4/CN-NG (0.4), and 3D NiFe2O4/CN-NG (0.4), which were applied in LIBs as anode materials, exhibited significantly high reversible capacities of 1032, 1105, 1008, and 919 mAh g−1 at current density of 0.1 A g−1, respectively. In particular, 3D MnFe2O4/CN-NG (0.4), as the anode material of LIBs, has maintained excellent magnification (capacity retention of 73%) and long-term stability after 800 cycles at the current density of 1 A g−1. The excellent electrochemical performance was mainly attributed to the unique 3D structure with some unique structural advantages. The layered structure was favorable for electrolyte penetration; N doping was beneficial to electron transmission by adjusting electronic structure. It had a large number of active sites and NPs were uniformly distributed; strong chemical bonds and metal carriers were conducive to improving lithium storage performance. Chen and colleagues141 used g-C3N4 nanosheets as the precursor and zinc powder as the template, from which the nitrogen-doped hollow carbon spheres (NHCS) were obtained. The NHCS delivered an acceptable initial discharge capacity of 1012.4 mAh g−1 at current density of 0.1 A g−1. It delivered promising cycling stability, which was 1064.5 mAh g−1 after 400 cycles as well as an excellent rate performance (360 mAh g−1 at 10 A g−1). Tin oxide and tin sulfide are widely studied by researchers for their characteristic conversion mechanism during the lithiating process, which endows them with high theoretical specific capacity. A simple approach promoting the performance of SnS2 was proposed here via strategically introducing the rGO/g-C3N4 hybrid to form a robust framework in the designed SnS2 composite. SnS2 was introduced in rGO/g-C3N4, which improved the conductivity of the SnS2 composite by Wen and colleagues.142 Excellent rate performance with a high recovered capacity of 864.9 mAh g−1 at current density of 800 mA g−1 was gained. The shuttle effect was efficiently suppressed by the N-rich host material. g-C3N4/carbon hybrid cages were rationally designed and fabricated as sulfur hosts. Chen and colleagues143 fabricated core-shell-structured S@g-C3N4/carbon cathode, which exhibited excellent cyclic stability. Ninety-one percent of the theoretical capacity of sulfur was achieved in the initial discharge at 0.2 C. The capacity of 636 mAh g−1, when S@g-C3N4/carbon served as the anode of LIBs, was obtained after 400 discharge/charge cycles at 1 C. Zuo et al.144 presented a one-pot solution-based strategy, in which SnS2 nanostructures were grown within a matrix of g-C3N4 and graphite plates (GPs). A series of SnS2-based LIB anodes had been researched and compared. When SnS2 nanoplates (SnS2-NPLs) were introduced in CN/GP, they provided the highest properties and displayed excellent rate capabilities (536.5 mAh g−1 at 2.0 A g−1), outstanding stability, and high pseudocapacitance contribution. The excellent electrochemical performance of SnS2/g-C3N4/GP composites materials is mainly attributed to the synergistic effects: porous g-C3N4 reduced the aggregation of SnS2 NPL, buffered the volume change of SnS2, and provided channels for Li ions; GP was conducive to charge transport; SnS2 NPL had a large comparative area for rapid Li ions intercalation and a proper geometry to stand volume expansions during lithiation/delithiation cycles. Cui and colleagues145 reported that a fluffy spherical Co1 − xS@g-C3N4 microcomposite, in which Co1 − xS NPs were uniformly anchored onto the cotton-like g-C3N4, was prepared via a facile one-step solvothermal method. The spherical-like Co1 − xS@g-C3N4 hybrid as anode material exhibited a higher reversible capacity of 789.59 mAh g−1 (at current density of 0.1 A g−1) than bare Co1 − xS (22.02 mAh g−1) after 210 cycles and delivered an ultralong cycle life of 1000 cycles with a capacity of 487.93 mAh g−1 (at current density of 0.1 A g−1). The excellent electrochemical performance of Co1 − xS@g-C3N4 was attributed to the following factors: (1) special fluffy structure; (2) the uniform adherence of Co1 − xS NPs to g-C3N4 matrix; and (3) buffer volume changes during charging and discharging processes. Transition metal dichalcogenides, particularly SnS2 with 2D layered structures analogous to that of graphite, had received special attention as innovative anode materials for practical applications. To improve low conductivity, the ND-g-C3N4 was fabricated via magnesiothermic denitriding method by Wang and colleagues.146 The high content of graphitic N will lead to serious irreversible capacity loss; the N content of g-C3N4 and successfully prepared ND-g-C3N4-n (ND-C3N4-n, n = heat treatment temperature) was reduced via magnesiothermic denitriding. ND-g-C3N4-675 presented excellent performance, which obtained 2264.9 mAh g−1 at current density of 1000 mA g−1 after 500 cycles. Amici and colleagues147 reported that g-C3N4 was directly used to synthesize SnO2@C3N4 for LIB anodes through a scalable solid-state reaction, in which anodes displayed high specific capacities of 1075 mAh g−1 at 156 mA g−1. SnO2@C3N4, as an anode in LIBs, achieved good rate capability and excellent stability upon prolonged cycling at high rates, which mainly attributed to regular and homogeneous distribution of SnO2 over g-C3N4 phase. The SnO2@C3N4 was easily obtained by a fast solid-state reaction by Amici and colleagues.147 The SnO2@C3N4 enabled the cell to achieve high rate capability (specific capacity of 1075 mAh g−1 at 156 mA g−1 with almost 50% of the initial specific capacity retained at current density of 3.9 A g−1) and excellent stability under prolonged cycling at high rates. As shown in Figure 7, Im and colleagues148 synthetized SnS2@g-C3N4 via a facile technique using a solid-state reaction. The obtained materials that demonstrated the specific capacity, rate performance, and cycling behavior were applied in LIBs. The excellent performance was mainly due to good dispersion of SnS2 nanosheets in the porous matrix of g-C3N4 and assigned to the buffering effect of the large surface area of the g-C3N4 matrix as well as the predominant contribution of the pseudocapacitive effect. g-C3N4, as a 2D functional material, has unique structural and electronic properties that are conducive to the storage of alkali metal ions and has attracted extensive research in the field of energy storage. It has a wide nitrogen-rich structure, a large triangular pore location, and a high theoretical LiB capacity of 524 mAh g−1. The lithium storage performance of g-C3N4, however, was greatly affected by its conductivity and structural performance. Hankel and colleagues149 successfully prepared Si-doped g-C3N4, which was an effective strategy to modulate its electronic properties and boost its reversible lithium storage capacity to solve the problem of ineffective lithium storage in monolayer 2D g-C3N4. The high performance included a lithium storage capacity of 557.7 mAh g−1. Mao and colleagues150 synthesized N-doped graphene as anode material for LIBs, for which g-C3N4 was used as a precursor with the assistance of polyvinylpyrrolidone (PVP). It had a promising specific capacity of 1236 mAh g−1 in LIBs at current density of 0.05 A g−1 and showed excellent cycling stability as well as acceptable rate capability.
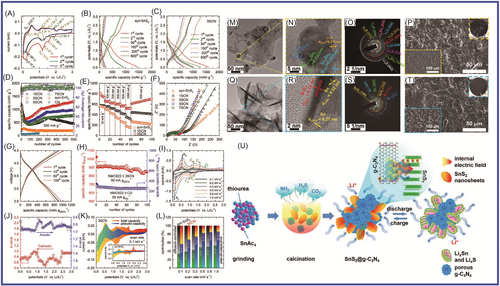
2D materials can provide a layered structure for transition metal oxides. CuO nanorods were grown on Cu/g-C3N4 nanosheets (where the 2D Cu nanosheets were grown on porous g-C3N4 nanosheets [Cu/g-C3N4]) (CuO@Cu/g-C3N4) by Su and colleagues.151 The CuO@Cu/g-C3N4 delivered a discharge-specific capacity of 726 mAh g−1 after 200 cycles at 0.1 C and a discharge-specific capacity of 457 mAh g−1 after 625 cycles at 1 C. Its outstanding stability and cycling performance were attributed to the unique 2D Cu nanostructure, which provided a large area and enhanced electronic transmission for Li+ insertion. The ordered CuO nanorods changed in volume for buffering during charging and discharging, providing more paths for charge and Li+ transfer, while the porous g-C3N4 nanosheets endow the prepared structure with more active sites. Pan and colleagues152 synthesized g-C3N4/Mo2CTx hybrid materials via a facile in situ strategy, which exhibited much improved Coulombic efficiency and cycling stability. The g-C3N4/Mo2CTx delivered excellent performance, including a reversible capacity of 229.6 mAh g−1 at 2 A g−1 after 1800 cycles, 528.5 mAh g−1 at 0.1 A g−1 after 100 cycles, and high initial Coulombic efficiency of 70.8%. Pan and colleagues153 reported that MoOx-UCN, where UCN is urea, in which MoO@MoO2 heterojunctions were anchored on 2D g-C3N4, had been synthesized, and the MoOx-UCN applied in LIBs showed excellent lithium-ion storage and rate performance (with a high capacity of 992 mAh g−1 under 0.5 A g−1 after 100 cycles and stable capacity of 716 mAh g−1 under 2 A g−1 after 500 cycles). Wang and colleagues154 prepared novel NGC nanosheet- (CN) and carbon nanotube (CNT)-encapsulated Co3O4 NPs (N-doped CN@Co-Co3O4/CNTs) via a simple self-template method, which exhibited high discharge capacity (460 mAh g−1 at 5000 mA g−1). Zhu and colleagues155 reported that N-doped multicavity Sn/C composite was prepared by a template-assisted structural design as an anode for LIBs, which displayed high specific capacity (512 mAh g−1 at 1.0 A g−1), good capacity retention (82% after 1000 cycles), and excellent rate performance. Vo et al.148 reported that the SnS2@g-C3N4 composite materials were produced by a facile one-pot synthesis method, in which the SnS2@g-C3N4 composite materials had different proportions of mixed precursors (different thiourea/SnAc4 mass ratios of 1, 3, 5, and 7) to identify the best proportion. The obtained SnS2@g-C3N4 samples were denoted as xSCN (x = 1, 3, 5, and 7). The 3SCN showed high performance (specific capacity of 1720.7 mAh g−1 at current density of 100 mA g−1), which was attributed to the buffering effect of the large-surface-area g-C3N4 matrix as well as the predominant contribution of the pseudocapacitive effect and the formation of heterointerfaces between the semiconductors SnS2 and g-C3N4. Pan and colleagues152 synthesized a g-C3N4/Mo2CTx hybrid, in which Mo2CTx was further exfoliated and the g-C3N4 protection layer was constructed by a facile in situ strategy. The g-C3N4/Mo2CTx delivered excellent Coulombic efficiency (70.8%) and good cycling performance (reversible capacity of 528.5 mAh g−1 at 0.1 A g−1 after 100 cycles) and cycling stability (229.6 mAh g−1 at 2 A g−1 after 1800 cycles). The high performance was mainly attributed to the unique structure of the g-C3N4/Mo2CTx hybrid, which increased the lithium storage performance, reduced the diffusion lengths of electrons and ions, and accelerated charge transfer. Lin and colleagues156 prepared hierarchical g-C3N4@WS2 composite material, which was intended for LiBs. It displayed outstanding lithium storage performance, with a specific capacity of 1136.1 mAh g−1 at 0.1 C, a high reversible specific capacity of 454.4 mAh g−1 after 200 cycles at 0.2 C, and superior cycling stability of 433.8 mAh g−1 after 1000 cycles. Chen and colleagues157 reported that the nitrogen vacancies in g-C3N4 were increased via energetic ion bombardment. The obtained ND-g-C3N4 (g-C3N4-x) delivered a high reversible capacity (high capacity of 647 mAh g−1 after 400 cycles at 0.1 A g−1) and stable cycling (ultrastable cycling performance of 232.8 mAh g−1 after 5000 cycles at 1 A g−1). The superior electrochemical performance was attributed to the increased specific surface, the reduced band gap, enhanced lithium absorption energy, different charge density due to the vacancy modulation, and active sites caused by the plasma bombardment. Sn/g-C3N4 (S/CN) composite, for which the capacity recovery of the S/CN composite was similar to that of Sn (437.8 mAh g−1) as current density was reversed to 100 mA g−1, was prepared using a simple chemical reduction method by Vo and colleagues.158
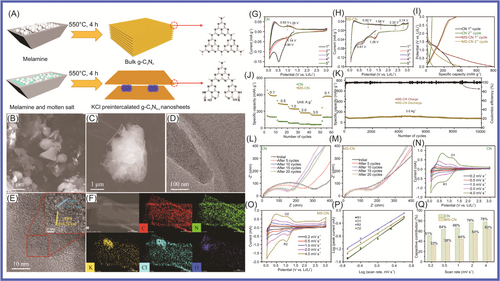
To improve the electrochemical properties of g-C3N4, Deng and colleagues159 prepared well-designed KCl preintercalated C3N4 nanosheets with abundant pyridinic-N, which showed a high specific discharge capacity and long cycle life (66% capacity retention after 10,000 cycles at 3.0 A g−1), via an one-step molten salt method, as shown in Figure 8. The KCl-preintercalated carbon nitride nanosheets applied in LIBs showed excellent electrochemical performance (high specific capacity and excellent cycle life), in which KCl as a prop enhanced the interlayer spacing and the structural stability, and the pyridinic-N and N vacancy increased the conductivity, as well as the reversibility of Li+ storage and the active sites. Dutta and colleagues160 studied the effect of g-C3N4 on the electrochemical performance of ZnS. ZnS/g-C3N4 composite anodes were obtained. One sample (0.7ZnS:0.3g-C3N4) delivered long-term reversible capacity (596.9 mAh g−1 after 1150 cycles in the Li+ half-cell configuration and 432.6 mAh g−1 after 750 cycles in the Na+ half-cell configuration, at a high current density of 1 A g−1). Dutta and colleagues161 prepared In2S3/g-C3N4 NC materials, which successfully enhanced performance. The best ratio was 0.8In2S3:0.2 g-C3N4, which delivered a reversible capacity of 358 mAh g−1/1000 cycles versus Li metal. Jin and colleagues162 synthesized spinel CuCo2O4 nanowire arrays and supported them on g-C3N4 nanosheets via facile hydrothermal methods, which formed a unique sandwich-like interconnected 3D mesoporous structure containing a high amount of void spaces. Tang and colleagues163 successfully prepared porous Si/nitrogen-doped CNS composites via in situ growth of nanosilicon on a graphene-like carbon. This material showed excellent electrochemical performance, 1106.7 mAh g−1 after 200 cycles with capacity retention of 93.6% and the Coulombic efficiency remaining above 99.5%. The fascinating surface chemistry of MXene allows the rational design of 2D heterostructures. Alshareef and colleagues164 designed a hybrid heterostructure (MXene@CN) comprising g-C3N4 and Ti3C2Tx. It was applied in LIBs, which showed excellent performance. This was mainly because MXene and g-C3N4 formed reinforcing bonds, and what is more, its chemical-bonding-induced charge redistribution ensured fast Li+ intercalation kinetics. Liu and colleagues165 synthesized a novel graphitic anode (Ni-g-C3N4) by using nickel (Ni) as the catalyst and g-C3N4 as the precursor. Ni-g-C3N4 demonstrated good performance (high capacity of 487.1 mAh g−1, with high capacity retention of 99.3% after 600 cycles and satisfactory average Coulombic efficiency of 99.4%).
2.2 g-C3N4 applied in LSBs
LSBs have attracted great attention as one of the most promising power sources due to their higher energy density, lower cost, and better environmental friendliness.166-169 LSBs have become one of the most promising electrochemical energy storage systems due to their high theoretical specific capacity (1673 mAh g−1) and high energy density (2600 Wh kg−1),170, 171 although there are issues with the polysulfide shuttle in LSBs.172 The problem of the polysulfide shuttle in LSBs leads to low Coulombic efficiency and cycle life, which has limited the practical application of LSBs.173, 174 Researchers have used hard carbon materials to combine with sulfur to promote electron transfer and inhibit the diffusion of polysulfides, thereby improving the stability of the electrode materials.175-178 The anchoring of polysulfides by nonpolar carbon interactions was weak, however, and could not inhibit the shuttling of polysulfides.179-182 g-C3N4, on the other hand, has abundant N content (pyridine nitrogen) and polar carbon and can utilize chemical adsorption to anchor polysulfides.
Meng et al.183 prepared g-C3N4 nanosheets, in which the nitrogen content was 56 wt% and the specific surface area was 209.8 m2 g−1, and sulfur was loaded onto them. The composite material, which had a sulfur content of 70.4 wt%, exhibited excellent specific capacity of 1250 mAh g−1 at 0.5 C and specific capacity of 578.0 mAh g−1 at 0.05 C during charge/discharge. The g-C3N4 contained abundant N atoms, which was beneficial for lithium sulfide loading and prevented the aggregation of sulfur, Li2S2, and Li2S. Gong et al.184 designed and prepared 3D lightweight and porous C3N4 nanosheets@rGO (PCN@rGO) materials, as shown in Figure 9. The specific capacities of PCN@rGO were 1205, 1150, 986, 800, 685, and 483 mAh g−1 at rates of 0.1, 0.2, 0.5, 1, 2, and 5 C, respectively. Li and colleagues185 synthesized porous g-C3N4 composed of graphite-like 2D corrugated sheets and high content of pyridinic nitrogen, which improved the performance of LSBs. The porous g-C3N4/S exhibited a specific capacity of 1050 mAh g−1 due to its unique porous structure and high charge polarity. The shuttle effect caused by the diffusion of polysulfides has limited the development of LSBs. g-C3N4 contains abundant pyridinic N, which is beneficial for the adsorption of polysulfides. Huangfu et al.186 covered a commercial polypropylene membrane with the ultrathin g-C3N4 membrane through vacuum filtration technology. The prepared material could effectively inhibit the shuttling of polysulfides. The prepared material exhibited an excellent reversible specific capacity of 829 mAh g−1 at 0.2 C after 200 cycles.
As shown in Figure 10A–D, the g-C3N4 was introduced into 3D graded porous graphene through molecular design to achieve polysulfide confinement and catalytic synergy by Wang et al.187 The hybrid material showed excellent specific capacities of 700 mAh g−1 (at current density of 0.5 C) and 600 mAh g−1 (at current density of 2 C), respectively. The hybrid material showed better sulfur utilization and cycle stability. The graphene network formed porous g-C3N4 nanosheets in situ, providing a surface to catalyze the conversion of polysulfides, and the porous carbon via assembly to provide channels for charge carriers. To solve the problem of the low conductivity of sulfur and sulfide shuttles, Chen et al.188 designed a functional diaphragm, which was coated with g-C3N4 and crystalline carbon (M-C3N4/C) on the surface of the commercial diaphragm. Transition metals were uniformly distributed on g-C3N4/C as active centers, which were chemically adsorbed on g-C3N4 to regulate polysulfide catalysis, facilitate rapid carrier transfer, and achieve efficient utilization of active sulfur species. The membrane-modified as-prepared Ni-C3N4/C hybrid material was applied in LSBs and showed a specific capacity of 999 mAh g−1 at 0.5 A g−1 and 724.6 mAh g−1 at 1.0 A g−1, respectively. It showed excellent cycling stability (with a high capacity retention rate of 89.4% after 300 cycles). As shown in Figure 10E–M, Zhou et al.189 prepared O/N co-doped hollow carbon microspheres (HCMs) using a sacrificial template method, which was obtained by carbonizing polydopamine. The as-prepared HCMs were constructed from porous nanosheets, with high specific area (873 m2 g−1), high pore volume (4.84 cm3 g−1), and high O/N doping (6.99 and 5.36 at%). The HCM-S hybrid material exhibited a specific capacity of 900 mAh g−1 at 1–2 C and excellent cycling stability (maintaining 530 mAh g−1 after 900 cycles). Deng et al.190 used SiO2 NPs as hard templates to synthesize graded porous g-C3N4/rGO through polycondensation and applied it in LSBs. The as-synthesized g-C3N4/rGO material had a sulfur content of 75% and a moderate macroporous density. The specific capacity was 958.5 mAh g−1 at 0.2 C. The g-C3N4/rGO hybrid material showed good cycling stability (specific capacity of 589.6 mAh g−1 at 2 C after 100 cycles). Its excellent electrochemical performance was attributed to the combination of its conductive rGO layer, polar g-C3N4, and graded porous structure with medium to large pore density in the main material. Fang and colleagues191 uniformly dispersed g-C3N4 on the electrode through a spraying method, which was a simple, time-saving, and universal method. Their sulfur graphene aerogel foam treated with g-C3N4 showed a specific capacity of 927 mAh g−1 at 0.2 C. After 280 cycles, it exhibited excellent cycling stability (with a specific capacity of 720 mAh g−1) after 280 cycles. The g-C3N4 nanosheet film slightly increased the quality of the electrode, thus inhibiting the “shuttle effect” of self-discharge and polysulfides. Jia et al.192 synthesized g-C3N4 through a simple method, which was used to synthesize active points anchoring polysulfides from trithiocyanuric acid to inhibit the shuttle effect. The synthesized g-C3N4 delivered a specific capacity of 1200 mAh g−1 at 0.2 C in LSBs. It could still maintain a specific capacity of 800 mAh g−1 with a Coulombic efficiency of 99.5% after 100 cycles. The synthesized g-C3N4 had high N doping, which was beneficial for improving the cycling stability of LSBs.
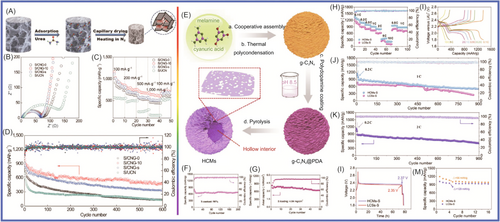
The separator was one of the research focuses of LSBs. Deng et al.193 prepared multifunctional molecular sieves, which were composed of g-C3N4, boron nitride, and graphene. The prepared molecular sieve crystal structure contains g-C3N4 with a channel size of 3 Å, which can effectively prevent the passage of polysulfides and facilitate the passage of Li+. BN served as a catalyst, and graphene facilitated charge collection and transport. The device exhibited a specific capacity of 600 mAh g−1 at 1 C after 500 cycles. With a sulfur loading of 6 mg cm−2, the specific capacity attenuation per cycle was less than 0.01%. Wu et al.194 prepared graphite-phase carbon nitride/CNT (g-C3N4/CNT) hybrid membranes through a vacuum filtration process. The as-prepared g-C3N4/CNTs could effectively adsorb and inhibit the shuttling of polysulfides. The prepared device exhibited a first discharge-specific capacity of 876 mAh g−1 at 0.5 C. The specific capacity remained 633 mAh g−1 after 300 cycles. The prepared hybrid material was conducive to adsorbing polysulfide and inhibiting self-discharge behavior. Sun et al.195 designed and synthesized mesoporous boron carbon nitride/graphene as a multifunctional modifier. The LSBs exhibited excellent cycling and high magnification capabilities. When the active material load was 4.6 mg cm−2, the device exhibited a specific capacity of 774.0 mAh g−1 at 200 mA g−1 after 80 cycles. When the active material load was 6.11 mg cm−2, the device exhibited a specific capacity of 573.6 mAh g−1 at 200 mA g−1 after 80 cycles. Guo et al.196 designed a layered structure to load sulfur. MnO2 nanosheets were grown on one side of carbon paper and g-C3N4/CNTs were loaded on the other side. The introduction of CNTs facilitated the infiltration of sulfur and electron transport. A g-C3N4@CNT-MnO2 hybrid material was obtained. When the sulfur content was 70.3 wt%, the device displayed an ultrahigh specific capacity of 1182.7 mAh g−1 at 0.2 C.
As shown in Figure 11A–F, Majumder et al.197 synthesized molybdenum disulfide/graphite-phase carbon nitride porous nanosheets (MoS2/g-C3N4) for application in LSBs. The as-prepared MoS2/g-C3N4 exhibited excellent cycling and rate performance (at the rate of 8 C, after 400 cycles, the specific capacity was 430 mAh g−1, with a decay rate of 0.028% per cycle). The excellent performance was attributed to the synergistic effects of lithium polysulfide with MoS2 and g-C3N4, which effectively inhibited the shuttling of polysulfides. Bai et al.198 prepared porous honeycomb-like C3N4 (PHCN) through the hard template method. PHCN-loaded S, as an electrode material applied in LSBs, exhibited an excellent high initial specific capacity of 1061.1 mAh g−1. The PHCN showed excellent rate performance, with a discharge-specific capacity of 495.1 mAh g−1 at 5 C. The specific capacity remained at 519 mAh g−1 at 1 C after 400 cycles. Under high load, the attenuation rate of each cycle was only 0.16% after 200 cycles. Ma et al.199 proposed a multiple lithium sulfide capture strategy, in which g-C3N4-x and CNTs were combined to form a hybrid material. The application of the g-C3N4-x/CNT modified separator in LSBs showed a discharge capacity of 1128 mAh g−1 at 0.2 C. The reversible capacity was 774 mAh g−1 after 100 cycles. Not only can the defect-rich g-C3N4-x catalyze polysulfide reactions with an affinity for polysulfides but also the g-C3N4-x/CNT hybrid material was conducive to electron transfer. Kim et al.200 designed an LSB interlayer with graded Fe3O4/C3N4 nanostructures to regulate the shuttle effect and improve sulfur utilization, as shown in Figure 11G–N. The synthesized Fe3O4/tubular (t)-C3N4 was beneficial for polysulfide anchoring to improve electron transport. t-C3N4 exhibited high surface area and anchoring ability to lithium polysulfide. The prepared Fe3O4/t-C3N4 was applied in LSBs, exhibiting an initial discharge capacity of 828 mAh g−1 at 2 C. The Fe3O4/t-C3N4 exhibited excellent cycling stability, with a specific capacity of 658 mAh g−1 after 1000 cycles (with a capacity decay of 0.02% per cycle). Wu et al.201 designed and synthesized a 3D-network-structured N-doped carbon material (C-NC) using biomass chitin as raw material. C-NC and GN were introduced into g-C3N4 to form C-NC/GN/g-C3N4 hybrid material. The as-synthesized S@C-NC/GN/g-C3N4 hybrid material in supercapacitors showed excellent cycling stability (with a specific capacity of 1130 mAh g−1 after 500 cycles). Tong et al.202 reported an additive, defective graphite-phase carbon nitride, which has a buffering effect on lithium polysulfide. The defect-rich g-C3N4-x combined with commercial polypropylene exhibited excellent performance.
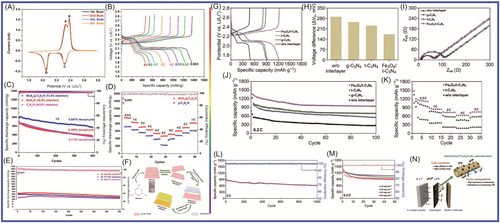
The interaction between graphite nitride carbon and Li2S plays a role in capturing polysulfides, as reported by Versaci et al.203 Their prepared device exhibited excellent performance, with a 25% increase in specific capacity and improved service life. Li et al.204 developed a porous structure with N-defect g-C3N4 (NDCN) for application in LSBs. The synthesized NDCN delivered an outstanding specific capacity of 620 mAh g−1 at 1 C after 300 cycles. After 300 cycles, each cycle was attenuated by 0.045% at 1 C. The prepared NDCN hybrid material had a unique 3D structure, with macropores and mesopores facilitating storage and carrier transport, as well as providing active sites. Pan et al.205 introduced sulfur into the interlayer between a TixOy-Ti3C2 layer and a C3N4 layer. A sandwich-structured oxytetraclycline/S/C3N4 material was formed. The prepared material was applied in LSBs, achieving a specific capacity of 749.5 mAh g−1 after 2000 cycles at 0.5 C. When the sulfur load was 4.2 mg cm−2, the LSB still maintained 70.5% of its initial specific capacity after 200 cycles. The interlayer structure of the material was conducive to LiPS anchoring, and the synergistic effect of the bidirectional interlayer enhanced the reduction kinetics of LiPS and Li2S. Wu et al.206 prepared porous g-C3N4 nanotubes (PCNNTs) using a template method as S carriers. The PCNNTs/S hybrid material was applied in LSBs, and the initial specific capacity was 704.8 mAh g−1. This excellent performance was attributed to the high specific surface area and abundant macropores/mesopores of PCNNTs, achieving high sulfur loading and buffering the volume changes during charging/discharging processes of PCNNTs with a tubular structure. To improve the performance of LSBs, Li et al.207 designed and prepared bimetal -doped Fe/Co C3N4/C materials to reduce the shuttling of polysulfides and improve their electrochemical performance. In LSBs, when the sulfur load was 1.8 mg cm−2, an initial specific capacity of 949 mAh g−1 was delivered at 0.2 C. After 135 cycles, the reversible specific capacity remained at 749 mAh g−1, with a decay rate of 0.156% per cycle. The Fe/Co-C3N4/C material had high specific surface area, high conductivity, and rich active sites, which improved the adsorption and conversion of lithium polysulfide. Zhang et al.208 prepared 3D porous-structured graphene-like C3N4 nanoflakes/carbon cloth/sulfur (g-C3N4/CC/S) composite material through simple thermal polymerization. The application of porous binderless g-C3N4/CC/S material in LSBs showed good electrochemical performance (with a specific capacity of 892 mAh g−1 at 0.2 C after 250 cycles). This excellent performance was attributed to the high porosity and favorable skeleton of g-C3N4/CC/S composite material for charge transfer. Carbon fiber was introduced to enhance conductivity. Wang et al.209 developed a g-C3N4/CNT hybrid material to enhance the multifunctional polysulfide barrier, inhibiting the shuttle effect and improving device performance, as shown in Figure 12. The obtained g-C3N4 had a unique sponge shape and a large specific surface area, providing carrier transport channels as well as an interface and active sites for polysulfide anchoring. The g-C3N4/CNT achieved excellent cyclability with a minimum decay rate of 0.03% per cycle (after 500 cycles). When the sulfur load was 10.1 mg cm−2, there was a high areal capacity of 7.69 mAh cm−2. Liu et al.210 prepared hollow tubular graphite-phase carbon nitride and applied it as a functional interlayer in LSBs to achieve the goal of suppressing the shuttle effect. It showed excellent performance (with a reversible specific capacity of 494 mAh g−1 at 1 C after 500 cycles). Each cycle had a specific capacity attenuation of 0.085%. N-doped tubular graphene-like (Tg)-C3N4 enhanced electron transfer and improved chemical adsorption and active sulfur catalysis. As a unique hollow tubular structure, Tg-C3N4 provided paths and active sites for ion migration. Jia et al.211 grafted carbon nitride onto waste-derived carbon. Sulfur grafted with C3N4-derived carbon was applied to LSBs, exhibiting a specific capacity of 1269.8 mAh g−1 at 0.05 C. The performance improvement was mainly attributed to the material's micropores, C3N4 as a sulfur carrier, and active sites on the surface. Xu et al.212 designed and prepared MoSe2@g-C3N4 composite material, MoSe2 loaded on the surface of g-C3N4 facilitated the migration of lithium polysulfide. When the MoSe2@g-C3N4 was applied in LSBs, it exhibited a specific capacity of 564.2 mAh g−1 at 3 C. At 0.5 C, the specific capacity decreased by 0.09% after 500 cycles at 0.5 C.
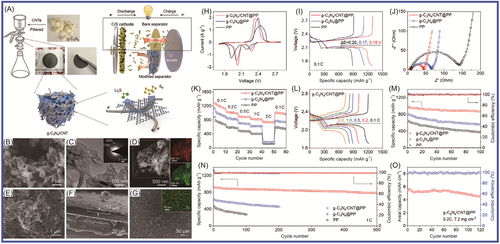
To improve the binding between nanosulfur and the main material, nanosulfur co-polymerization was achieved through a solution pathway under the action of 1,3-distyrene monomer. The loading of sulfur NPs on carbon-rich GCN was found to be beneficial for charge transfer by Tiwari et al.213 The sulfur content of the prepared composite material was 76%, indicating an initial specific capacity of 1380 mAh g−1 at 0.1 C. The discharge capacity was 700 mAh g−1 at 1 C after 1000 cycles. Each cycle had a specific capacity attenuation of 0.016%. Liu et al.214 uniformly dispersed and anchored CoS onto g-C3N4 nanosheets, forming CoS@g-C3N4 hybrid material. The as-synthetized CoS@g-C3N4 was loaded on polypropylene film. The device exhibited excellent performance with a specific capacity of 1290 mAh g−1 at 0.2 C. It delivered excellent cycling stability (with a specific capacity of 600 mAh g−1 at 0.1 C after 250 cycles). Wang et al.215 prepared protonated g-C3N4 (pCN)/CNTs by dissolution precipitation method. pCN was dispersed in H2SO4 and electrostatically self-assembled with CNTs. The optimal ratio of pCN to CNT was 1:1 (CC 1:1), exhibiting a specific capacity of 711.9 mAh g−1 at 1 C after 500 cycles. Sulfur was loaded on g-C3N4 and rGO, and PPy was polymerized and coated on the composite material. Moon et al.216 prepared g-C3N4/rGO/S@PPy hybrid material for application in LSBs. It showed a specific capacity of 1189 mAh g−1 after 200 cycles. When the sulfur content was 5 mg cm−2, the displayed g-C3N4/rGO/S@PPy areal capacity was 5.5 mAh cm−2. He et al.217 prepared graphite-phase carbon nitride and functionalized CNTs (g-C3N4/CNTs) through calcination and self-assembly methods, forming a 3D hybrid sulfur host. g-C3N4 nanosheets were grown in situ on the surface of CNTs and bonded with C–N bonds to form a large π-conjugated system. The hybrid material had a high sulfur utilization rate and polysulfide interaction, which effectively inhibited the polysulfide shuttle. The sulfur content was 80 wt%, and the device delivered a high specific capacity of 1351.2 mAh g−1. When the sulfur load was 5 mg cm−2, the initial specific capacity was 758.9 mAh g−1. After 200 cycles, the specific capacity retention rate was 77.1% at 1 C.
2.3 g-C3N4 applied in SIBs
SIBs are considered promising candidates to partially replace LIBs due to their abundant sodium resources and similar ion storage mechanisms.218-220 Their lower energy/power density and poor cycling stability have hindered their practical application, however.221-223 Increasing the surface load of active substances could effectively improve the energy/power density of SIBs.47, 224-226 The development of high-performance, inexpensive, and easily available negative electrode materials is of great significance for the industrialization of SIBs.227, 228
Lu and colleagues229 prepared antimony/graphitic carbon composite via a facile, high-throughput ball-milling method. The antimony/graphitic carbon composite materials were tested as anode for SIBs, which delivered an excellent overall performance in terms of packing density (200 mAh g−1 at 20 C), fast charge/discharge capability, and cyclability. The graphite matrix, which was tough and dense, provided an electron transport channel for the active materials, and the buffered antimony anode collapsed during charging and discharging. The optimization of graphite properties further improved the performance by increasing the cycle reversibility. Ultrathin N, P dual-doped hollow carbon fibers/g-C3N4 (huCP/g-C3N4) was synthetized by a simple and scalable approach, using common filter paper as a precursor, by Yang and colleagues230 As anode for SIBs, the huCP/g-C3N4 provided high reversible capacity of 345 mAh g−1 after 380 cycles at current density of 0.1 A g−1 and high capacity of 110 mAh g−1 after 4000 cycles at current density of 1 A g−1. The huCP/g-C3N4 and g-C3N4 networks had high contents of doped N and P, which not only had more defects and active sites but also expanded the plane surface area and provided conductive networks. Finally, the huCP/g-C3N4 as electrode material in SIBs showed excellent electrochemical performance. Camean and colleagues231 reported that graphitic carbon foam was prepared from bituminous coal via a simple procedure to construct suitable anode materials for SIBs in glyme-based electrolytes. The graphitic carbon foam was applied in SIBs, which exhibited excellent electrochemical performance (up to 90 mAh g−1 after 300 cycles at high electrical current density of 1.9 A g−1 with a Coulombic efficiency of 100%). Na+ ions underwent different combined intercalation processes through pseudocapacitance intercalation and diffusion control. The increased graphene layer spacing and the presence of boron promoted pseudocapacitance intercalation, and the increase in controlled intercalation capacity was mainly related to the increased boron content. Cao and colleagues232 reported that the N/S-co-doped hierarchically PCSs (denoted as S-N-HPCS), which had a large specific surface area (904.63 m2 g−1) and a high heteroatom-doping level (N: 10.51 wt%; S: 1.71 wt%), were synthesized by a novel and simple self-sacrificing dual-template strategy. g-C3N4 formed in situ and amorphous ZnO were used as sacrificial templates for preparing HPCS via high-temperature pyrolysis. The S-N-HPCS exhibited excellent reversible capacity of 270.1 mAh g−1 at current density of 100 mA g−1 and high stability (160.1 mAh g−1 after 2000 cycles at 1 A g−1 with a capacity retention of 82.3%). The spacing of g-C3N4 layers was limited, which limited the storage of sodium ions in the lattice. Highly ordered sulfur-doped MCN (S-MCN) was synthetized through a hard template approach by employing dithiooxamide as a single molecular precursor containing carbon, nitrogen, and sulfur elements by Vinu and colleagues.233 The larger S ions were introduced into the MCN lattice, and the interlayer spacing of carbon nitride was significantly expanded. This endowed the lattice with high capability for Na ion accommodation. Vinu and colleagues demonstrated via the first-principles density functional theory (DFT) calculations that the present S-MCN was highly optimized not only in terms of its chemical structure but also for taking up abundant Na ions with high adsorption energy. The optimized samples delivered a high reversible capacity of 304.2 mAh g−1 at current density of 100 mA g−1 after 100 cycles with nearly 100% Coulombic efficiency as an anode material for SIBs, which was far superior compared to the performances of nonporous S-CN and g-C3N4. Compared to the nonporous S-CN (167.9 mAh g−1) and g-C3N4 (5.4 mAh g−1), the specific discharge capacity of the SIBs was 304.2 mA h g−1. This excellent electrochemical performance was mainly attributable to the increased interlayer spacing, highly ordered structure, large surface area, uniform pore size distribution, and significant surface-induced capacitive contribution. Expanding the layer spacing revealed the influence of atomic doping on the structure of carbon materials, which became more effective anode materials for SIBs. Qiao and colleagues234 reported that a series of N-rich few-layer graphene (N-FLG) samples had been successfully synthesized with tunable interlayer spacing ranging from 0.45 to 0.51 nm via annealing g-C3N4 with zinc catalysis at selected temperatures (T = 700°C, 800°C, and 900°C), as shown in Figure 13. Due to the stronger electrostatic repulsion of pyrrole N, the interlayer spacing of graphene was increased. The N-FLG-800, which was used in SIBs as anodes, exhibited the optimal properties in terms of interlayer spacing, nitrogen configuration, and electronic conductivity. It showed excellent long-term stability (211.3 mAh g−1 at 0.5 A g−1 after 2000 cycles) and ultrahigh rate capability (56.6 mAh g−1 at 40 A g−1).
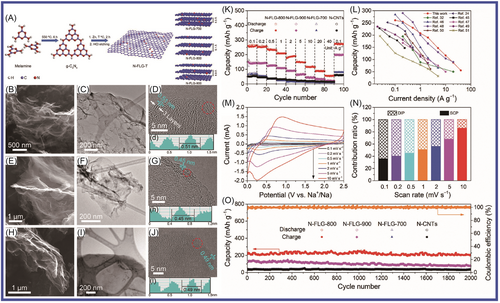
To improve g-C3N4 performance in SIBs, Taylor and colleagues235 prepared multilayer C/g-C3N4 composites via a facile one-pot heating of a mixture of low-cost urea and asphalt for application in SIBs, as shown in Figure 14A,B1–B6,C1–C4. The C/g-C3N4 demonstrated a specific capacity of 254 mAh g−1 (about two times higher than that of g-C3N4), and high Coulombic efficiency (~99.8%), rate capability, and cyclability. The discharge capacity remained at about 120 mAh g−1 after 14,000 cycles at 1 A g−1. Molaei et al.236 studied the application of P-doped g-C3N4 in SIBs using DFT. The results showed that P-doping narrowed the band gap of g-C3N4. The adsorption of Na on P-doped g-C3N4 was studied by investigating the density of states. The pyridinic N atom in g-C3N4 played a major role in the adsorption of Na. The calculations showed that when more P was doped, the lower voltage (below 2.2 V) was favorable for Na diffusion (with a barrier of 1.2 eV). The results showed that P-doped g-C3N4 was more suitable as an anode material for SIBs. As shown in Figure 14D1–D11,E1–E6, Xiao and colleagues237 deposited ultrathin g-C3N4 on Cu metal electrode and studied its effects on Na storage. The g-C3N4 film, which was prepared via a facile, efficient, and general chemical vapor deposition (CVD) method, was as thin as 10 nm. It delivered high reversible capacity up to 510,00 mAh g−1. This indicated that sodium was stored in g-C3N4, which was favorable for activating metal and sodium deposition. At the same time, an SEI layer was formed to avoid metal contact with the liquid electrolyte. Na2Ti3O7, which has the two disadvantages of low conductivity and sluggish Na+ diffusion in interfacial reactions as electrode material for SIBs, has stood out among many materials because of its low discharge voltage platform at about 0.3 V (relative to Na/Na+) and low cost. He and colleagues238 reported that Na2Ti3O7 nanotube species had been anchored to composites composed of graphite-phase carbon nitride (g-C3N4) and ultrafine graphene via a facile strategy (alkaline hydrothermal), forming 3D network structures. One Na2Ti3O7NT/g-C3N4/rGO sample retained a capacity of 210.8 mAh g−1 (after 300 cycles at 0.1 A g−1) and good rate capability (104.7 mAh g−1 at 2 A g−1) as a sodium storage material. The excellent electrochemical performance of Na2Ti3O7 nanotube/g-C3N4/graphene was mainly attributed to the following factors: the Na2Ti3O7 nanotubes in these carbon matrices could effectively shorten the Na+ transport paths, and g-C3N4/rGO support caused more active sites for Na+ insertion/extraction and accommodated the volume expansion of Na2Ti3O7.
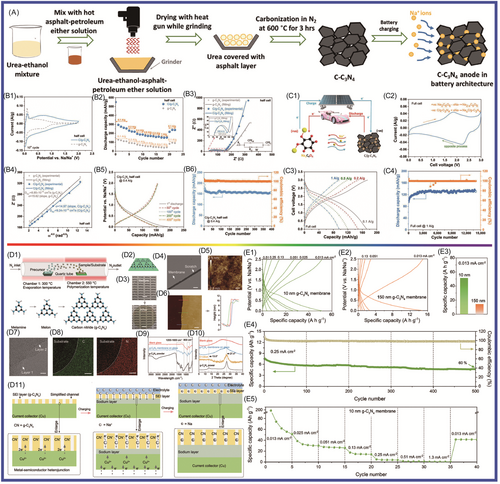
MoS2 as an anode material for SIBs has attracted extensive attention from researchers. Its poor electronic conductivity and cycling stability have limited its application. Zhou et al.239 reported that MoS2 nanosheets supported on ND-g-C3N4 were synthesized by a simple hydrothermal method. ND-g-C3N4 was obtained by magnesiothermic denitriding technology. Due to the synergistic effect between the superior electronic conductivity of ND-g-C3N4 and the ultrathin MoS2 nanosheets, the MoS2-ND-C3N4 as anode for SIBs exhibited excellent initial reversible specific capacity of 610.66 mA h g−1 at 0.1 A g−1, remarkable long-term cycling stability with 365.8 mA h g−1 at 1 A g−1 after 500 cycles, and superior rate performance. The structure of MoS2-ND-C3N4 was ingeniously designed. Due to its nitrogen-doped carbon materials, ND-g-C3N4 showed excellent electronic conductivity, which could effectively enhance the diffusion mobility of Na+ and provide rapid electron transfer, avoiding MoS2 agglomeration, and shortening the diffusion distance of Na+. The application of MoS2 in SIBs has been limited by its layered structure and stacking performance. To improve its cyclability and rate performance for Na storage, 3D hierarchical g-C3N4/graphene-supported covalent assembly of MoS2-SnS (MoS2-SnS@g-C3N4/G) composite materials was synthesized by hydrothermal treatment by Zhao and colleagues.240 The MoS2-SnS@g-C3N4/G displayed excellent rate capability of 452 mA h g−1 at 5 A g−1 and high cycling stability of 320 mA h g−1 at 2 A g−1 with 54.7% capacity retention after 500 cycles as an anode material for SIBs. Its excellent performance was attributed to the unique design of its material structure, which not only enhanced the conductivity to promote electron transport at the interface (which was verified by experiments and DFT) but also buffered the volumetric changes of MoS2-SnS. Carbon materials have attracted extensive attention from researchers and are widely used as anode materials for Li+/Na+/K+-ion batteries. The intercalation and desorption of carbon layers need different layer spacing due to different radii of alkali metal ions. N-doped graphene, which offered tuned interplanar spacing, was synthesized using g-C3N4 as a precursor with the assistance of PVP by Mao and colleagues.150 The N-doped graphene had a high nitrogen doping level, especially pyrrolic nitrogen, which led to adjustable layer spacing in the range of 0.34–0.45 nm. As an anode for SIBs, the N-doped graphene delivered a promising specific capacity of 300 mAh g−1 at current density of 0.05 A g−1 and excellent cycling stability as well as acceptable rate capability. This was due to the lower energy barrier for Na+ ions intercalation, which could be reduced significantly in expanded interlayers induced by the presence of pyrrolic nitrogen doping. The larger interplanar distance was beneficial to the storage of the Na+ ions with larger radius. To study the “closed pore” controversy relating to carbon materials in sodium storage, Oschatz and colleagues241 reported polymeric carbon nitride on hard carbon fibers that were prepared with both open and closed microporosity via CVD. The results showed that the ordered graphene layer exhibited a specific capacity of 220 mAh g−1 for SIBs. g-C3N4 has attracted extensive attention due to its high theoretical specific capacity and chemical stability. The actual capacity of g-C3N4 was very low, however, due to the damage of its crystallinity from the intercalation and agglomeration of metal ions. Li and colleagues242 prepared a crystalline C3N4-based carbonaceous composite via a molten-salt synthesis protocol for the first time, as shown in Figure 15, which was designated as MSGM. Carbon addition, for which glucose is used as the prototype carbon source, could direct the growth of C3N4. The as-prepared crystalline C3N4/C exhibited a record-high reversible capacity of 286 mAh g−1 at current density of 0.05 A g−1 and 122.8 mAh g−1 at 2 A g−1 for 2000 cycles without any attenuation. This excellent electrochemical performance was attributed to the following factors: crystalline C3N4 could increase the charge transfer rate through Na+ intercalation; the 2D nanosheets had rich edges and pores, which could accommodate more Na+, effectively avoiding the agglomeration of carbon and C3N4; and the high concentration of pyridinic nitrogen reduced irreversible reactions and improved Na+ storage performance. Wang and colleagues243 fabricated a novel hybrid of amorphous Sb/N-doped layered carbon (a-Sb/NC), in which ultrafine a-Sb NPs were uniformly anchored on layered N-doped carbon, via a facile bottom-up method, using C3N4 as both a reduction agent and a carbonaceous scaffold. The as-prepared a-Sb/NC, as anode for SIBs, delivered a large reversible capacity of 479.6 mAh g−1 at current density of 0.1 A g−1 after 100 cycles, robust rate capability of 298.7 mAh g−1 at current density of 2 A g−1, and cycling stability of 280.5 mAh g−1 at current density of 1.0 A g−1 after 500 cycles. In the a-Sb/NC materials, the layered NC prepared from C3N4 provided support for a-Sb NPs grown in situ and also provided channels for electron/ion transport. At the same time, the isotropic characteristics and short-range ordering of the amorphous structure were conducive to Na+ intercalation, promoting diffusion and improving activity. Dutta et al.160 reported that ZnS/g-C3N4 composite materials were synthesized by an expandable acid-assisted sonochemical method, and the ZnS NPs were dispersed in g-C3N4 nanosheets. This effectively allows the combination of high capacity ZnS with modified g-C3N4 support, which has a large amount of pyridinic nitrogen to assist in enhanced Li+/Na+ storage and thus leads to a synergistic effect, improving its electrochemical performance and stability. The 0.7ZnS:0.3g-C3N4 composite material exhibited excellent electrochemical performance and delivered a reversible capacity of 583 mAh g−1 after 100 cycles for SIBs at a current rate of 0.1 A g−1. The as-prepared 0.7ZnS:0.3g-C3N4 composite material showed a reversible capacity of 432.6 mAh g−1 after 750 cycles at a high current density of 1 A g−1 in SIBs. ZnS was loaded on g-C3N4 (with a large amount of pyridinic nitrogen), which was conducive to enhancing Na+ storage. The well-distributed SnS2 nanosheets on g-C3N4, which was synthetized from tin(IV) acetate and thiourea, was prepared via a mass-scalable and facile one-pot method by Im and colleagues.244 The synthesized SnS2@g-C3N4 composite served as an anode-active material for SIBs with outstanding performance in terms of high capacity and ultralong cycling stability (919.6 mAh g−1 after 400 cycles at current density of 500 mA g−1; 602.7 mAh g−1 after 3000 cycles at current density of 2000 mA g−1). The excellent electrochemical properties of the obtained composites were mainly attributed to the following advantages: g-C3N4 was used as the support material to effectively mitigate the volume changes of SnS2 during charging and discharging. SnS2, which had a large layer spacing, and g-C3N4 formed a self-induced internal electric field at the heterogeneous interfaces, which was conducive to the transmission of electrons and sodium ions and improved the electrochemical performance. Red phosphorus/rGO-C3N4 (P/rGO-C3N4), in which N-enriched rGO-C3N4 hybrid network was proposed as a matrix for phosphorus composites, was synthesized as anode for SIBs via a facile ball-milling method by Cui and colleagues.245 The interfacial compatibility between rGO-C3N4 and red phosphorus was realized by P–N single bonds. At the same time, the interface bonding was revealed through theoretical calculations. Based on the first-principles calculations, it was shown that rGO-C3N4 achieved more band-gap overlap near the Fermi level than C3N4, which was conducive to the electron transfer between the rGO and the C3N4 interface, and ultimately improved the electrochemical performance of the composite. The P/rGO-C3N4 delivered an excellent discharge capacity of 652.6 mAh g−1 after 100 cycles as anode for SIBs. To improve the electrochemical kinetics and structural collapse of TiO2 during charging/discharging, He and colleagues246 prepared TiO2 nanotubes anchored on g-C3N4/rGO hybrid nanosheets (TiO2NT/g-C3N4/rGO) as anode for SIBs by an alkaline hydrothermal method. The synthetized TiO2NT/g-C3N4/rGO demonstrated high reversible capacity of 138.5 mAh g−1 after 244 cycles at 0.1 A g−1 and good rate capability of 103.3 mAh g−1 at 0.8 A g−1. The synergistic effect of highly conductive graphene and porous g-C3N4 provided ready channels for the rapid diffusion of ions/Na+ and also provided sufficient conductive paths for effective charge transfer. The TiO2 nanotubes were uniformly distributed on the g-C3N4/rGO, which buffered the agglomeration and prevented structure collapse of the TiO2 nanotubes during charge/discharge cycling. Wu and colleagues247 reported that nitrogen-containing functional groups could be regulated by constructing g-C3N4/graphite heterojunctions via a simple and effective ball-milling strategy based on mechanochemistry. To promote the sodium storage capacity, defect-enriched g-C3N4/graphite heterojunctions were formed, and the nitrogen-containing functional groups were regulated during the ball-milling process. The prepared g-C3N4/graphite delivered excellent long cycling stability (202 mAh g−1 at current density of 1.0 A g−1 after 6000 cycles and 90.06 mAh g−1 at current density of 5.0 A g−1 after 10,000 cycles, respectively) and rate performance. The g-C3N4/graphite heterojunctions, in which the buckled g-C3N4 offered high energy for sodium adsorption and the graphene sheets acted as a low energy barrier for sodium diffusion, showed the synergistic effect of sodium ion adsorption and diffusion. N, P, O ternary-doped mesoporous carbon (PNPOC-T, T = 700°C, 800°C, or 900°C) with large interlayer spacing (about 0.4 nm) was synthesized by in situ polymerization and carbonization as anode material for sodium ion hybrid capacitors by Lv and colleagues,248 as shown in Figure 16. PNPOC-800, which had abundant heteroatoms and a high mesopore/micropore volume ratio, exhibited excellent rate performance (250.1 mAh g−1 at current density of 0.05 A g−1 and 101.5 mAh g−1 at current density of 20 A g−1, respectively). The obtained PNPOC-800 presented a high power density (13.59 kW kg−1) and long-term cycling stability (reach 87.43% after 9000 cycles at 1 A g−1). The PNPOC-800 had large interlayer spacing (about 0.4 nm), indicating a high mesopore/micropore volume ratio, was conducive to Na+ storage, promoted electronic conduction, and reduced ion transfer barriers. g-C3N4@carbon composite (CCN) was prepared via a simple gel coating–calcination method by Long and colleagues.249 The CCN exhibited a high discharge capacity of 250 mAh g−1 at current density of 0.1 A g−1 and 183 mAh g−1 at current density of 3 A g−1, respectively. It delivered long-term cycling performance with capacity of 180 mAh g−1 over 1600 cycles at current density of 0.5 A g−1. This excellent electrochemical performance was due to the increased pyridinic N content and electron transport, as well as the unique fluffy porous structure. Singh and colleagues250 reported that RGO and g-C3N4@rGO were synthesized by the hydrothermal method for SIBs, in which RGO was prepared by the hydrothermal method and was further soaked in saturated urea solution, followed by calcination to synthesize g-C3N4@rGO composites. The prepared g-C3N4@rGO composite material as anode exhibited a maximum reversible specific discharge capacity of 170 and 160 mAh g−1 at current density of 0.1 C and at current density of 0.2 C, respectively. The g-C3N4@rGO composite electrode showed reversible specific capacity of 176 mAh g−1 at current density of 0.2 C with 100% Coulombic efficiency, even after 2000 cycles. The submicron porous structure of g-C3N4@rGO could effectively prevent the accumulation of RGO nanosheets and provide channels for the insertion and removal of Na+. As shown in Figure 17, Lu et al.104 reported that fullerene-intercalated g-C3N4 (C60@CN) hybrid material was synthesized by facile thermal polymerization. The C60 featured in situ binding to g-C3N4 nanosheets (forming C60@CN composite material), which was conducive to enhancing electronic transmission, and with the layer spacing ranging from 0.33 to 0.42 nm protected the g-C3N4 from agglomeration during charging and discharging. This structure was conducive to the exposure of edge N defects (pyridinic N and pyrrolic N) and increased sodium ion storage. The C60@CN composite material, as anode materials for SIBs, delivered a high reversible capacity of 430.5 mAh g−1 at current density of 0.05 A g−1 (about three times higher than that of pristine g-C3N4), excellent rate capability of 226.6 mAh g−1 at current density of 1 A g−1, and ultralong cycling performance (101.2 mAh g−1 after 5000 cycles at current density of 5 A g−1).

2.4 g-C3N4 applied in PIBs
With the rapid development of electric vehicles and large-scale energy storage systems, the consumption of lithium resources has also accelerated, which has led to a gradual increase in the price of LIBs.251-253 To meet the growing demand for energy storage equipment, the emergence of PIBs has provided researchers with new ideas.254, 255
Sun and colleagues256 reported that they had fabricated nitrogen-doped porous CNSs (N-PCSs) from petroleum coke with an ultrahigh nitrogen content (about 11.7%) by a facile template-assisted approach using g-C3N4 as both a template and nitrogen source. The prepared N-PCSs showed excellent electrochemical performance (206 mAh g−1 after 300 cycles at current density of 1000 mA g−1) in PIBs. Their excellent electrochemical performance was attributed to the unique microstructure of carbon-based materials (nanoflakes, high porosity, high nitrogen doping, and high degree of disorder). To better study fast transmission of K+, high specific capacity, and power, different materials were investigated. Zhang and colleagues257 designed and prepared one-dimensional (1D)/2D C3N4/rGO composite as anode material, as shown in Figure 18A,B1–B9,C1–C6, which achieved a specific capacity of 464.9 mAh g−1 after 200 cycles at 1 A g−1 and 228.6 mAh g−1 after 1000 cycles at 10 A g−1, respectively. Composite materials combine multiple electrochemical advantages: structural stability and the synergistic interaction between 1D C3N4 and 2D rGO, a large surface area for additional active sites, and shorter K+ diffusion distance. Potassium-based energy storage devices have received extensive attention from researchers due to the abundant resources and high mobility of potassium ions. Carbon-based materials exhibit great potential in potassium ion storage, but their electrochemical performance and cycle life are poor. Nitrogen doping has been proven to be an effective modification strategy for improving the electrochemical performance of carbon materials. Shen et al.106 synthesized carbon nanomaterials (C3N4@NCNF-600, where NCNF stands for nitrogen-doped carbon nanofiber) in Figure 18D,E,F1–F5,G1–G4,H1–H6. During the annealing process, thiourea could be decomposed into g-C3N4 and embedded into the carbon fibers, greatly increasing their nitrogen content (with the sum of pyrrolic N and pyridinic N as high as 59.51%). As anode for PIBs, C3N4@NCNF-600 delivered high specific capacity of 391 mAh g−1 at current density of 0.05 A g−1, rate capacity of 141 mAh g−1 at 2 A g−1, and long cycling capacity of 201 mAh g−1 at 1 A g−1 over 3000 cycles.
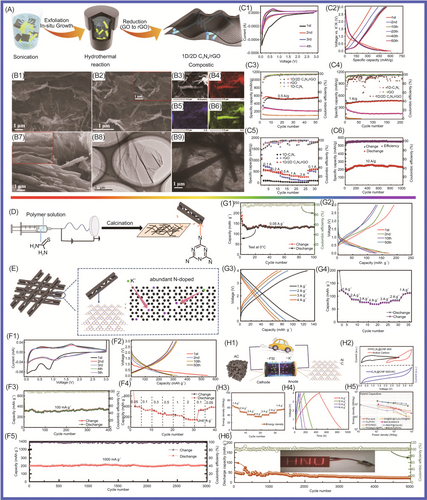
2.5 g-C3N4 applied in supercapacitors
Supercapacitors have the advantages of high power density and fast discharge speed, and are widely used for energy storage.258-262 At present, most pseudocapacitive materials (such as nickel hydroxide) in supercapacitors have poor cycling stability and small specific surface areas.263-265 g-C3N4, as a special nitrogen-doped carbon material, has rich nitrogen content and can enhance surface polarity and improve surface wettability.266, 267 It is easy to graft with other materials due to its highly crystalline layered structure, transfer of charges along the horizontal direction, and large surface area. It can offer more active sites, improve the electron acceptor/donor properties, and provide additional pseudocapacitance.268-272 g-C3N4 was used to form a composite with other pseudocapacitive electrode materials (metal oxides/hydroxides, etc.), which can improve the specific capacitance and cycling stability of the pseudocapacitive electrode.273, 274
Wen et al.275 reported that crumpled NG nanosheets (C-NGNSs), which had pore volume as high as 3.42 cm3 g−1, were fabricated via an efficient and facile strategy. The C-NGNSs-900 sample, which possessed a specific capacitance of approaching 248.4 F g−1 at 5 mV s−1, had the highest capacity. Its specific capacity was four times higher and two times higher than the specific capacities of TRGSs (51.7 F g−1) and CRG (106.3 F g−1), respectively. The excellent performance parameters (such as capacity, rate, and cycling stability) displayed in these supercapacitors were attributed to the unique pleated structure, high pore volume, nitrogen doping, and excellent conductivity of C-NGNS-900. Graphitic-C3N4 nanofibers (GCNNFs) were synthesized via a facile and efficient method that was amenable to scaling up by Tahir et al.276 The GCNNFs were applied as supercapacitor electrode, which exhibited high capacitance of 263.75 F g−1 and excellent cycling stability in 0.1 M Na2SO4 aqueous electrolyte with capacitance retention of 93.6% after 2000 cycles at current density of 1 A g−1. The as-synthesized GCNNFs with high surface area, which was favorable for higher conductivity and electrochemical performance and provided a large electrode-electrolyte contact area, had 1D structure with a higher concentration of nitrogen. The poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT) and g-C3N4 with specific capacitance of 137 F g−1 in H2SO4 and 200 F g−1 in Na2SO4 at current density of 2 A g−1, was prepared via a layer-by-layer assembly method by Chen et al.277 Its excellent performance was attributed to the synergistic effects of the two components in the composite. Nickel hydroxide has attracted widespread attention due to its high theoretical capacity, but its short cycle life has limited its application. Ding and colleagues278 prepared g-C3N4/Ni(OH)2 composites by a one-step method, wherein Ni(OH)2 had an interconnected honeycomb structure. Compared with g-C3N4 (416.5 F g−1 at 7 A g−1) and Ni(OH)2 (968.9 F g−1 at 7 A g−1), g-C3N4/Ni(OH)2 composite exhibited higher specific capacity (1768.7 F g−1 at 7 A g−1 and 2667 F g−1 at 3 mV s−1, respectively) and cycling stability (84% capacity retention after 4000 cycles). This excellent performance was attributed to the chemical stability of the material and its unique structure, which promoted vertical charge transfer. As shown in Figure 19A–N, Li and colleagues279 reported that carbon self-repairing porous g-C3N4 nanosheets (CPCN-NSs), which had a high surface area (220.7 m2 g−1), had been prepared by a solvothermal process coupled with a multistep post-thermal treatment in air. Then, CPCN-NSs/NiCo2S4 NPs (CPCN-NSs/NCS-NPs) hybrid composite materials were synthetized via the hydrothermal method. Through electrochemical performance testing, the prepared CPCN-NSs/NCS-NPs composite material exhibited excellent specific capacity (1557 F g−1 at current density of 1 A g−1) and cycling stability (only 7.4% loss after 10,000 cycles). Its excellent performance was attributed to several advantages: the preparation of CPCN-NSs with a large surface area, ultrathin thickness, and porous structure, which could provide a good scaffold for NCS NPs; and NCS NP, which had high conductivity, was used as a conductive connector between the CPCN-NSs to improve the conductivity of the hybrid composites. Li et al.280 reported that NCS nanosheets/porous g-C3N4 nanosheets (NCS NSs/P-g-C3N4) had been prepared via the hydrothermal method, by which NCS nanosheets were uniformly anchored on porous g-C3N4 nanosheets. NCS NSs/P-g-C3N4 exhibited high specific capacity (506 C g−1 at a current density of 1 A g−1), outstanding rate capability, and excellent cycling stability (about 99% capacity retention after 5000 continuous cycles). NCS NSs/P-g-C3N4 was combined with activated carbon (atomic cluster [AC]) (NCS NSs/P-g-C3N4//AC) to construct an asymmetric supercapacitor, which delivered high specific energy (16.7 Wh kg−1 at a specific power of 200 W kg−1).
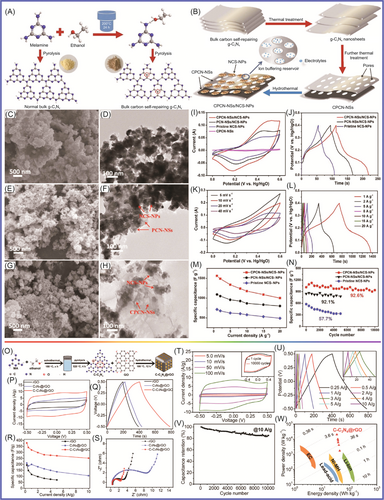
To improve the charge mobility of g-C3N4, Ding et al.281 prepared carbon self-repairing g-C3N4 (C-C3N4), as shown in Figure 19O–W, which was self-assembled with graphite oxide to form a porous structure. The as-prepared C-C3N4@rGO delivered the high specific capacity of 379.7 F g−1 and energy density of Wh kg−1 at 0.25 A g−1. Its excellent electrochemical performance was attributed to the following factors: in self-repairing C-C3N4, N replaced C to form C–N bonds, which went on to form large delocalized π bonds. The π-conjugated plane layers were in close contact with the GO, forming a 3D structure that facilitated electron migration and electrolyte infiltration. Zhang et al.282 reported that Ni2P2O7 nanoarrays grown on Ni foam with decorated C3N4 thin nanosheets (C3N4/Ni2P2O7) on its surfaces were achieved via a hydrothermal and in situ calcination strategy. The optimized C3N4/Ni2P2O7 composites delivered pseudocapacitance as high as 2.7 F cm−2 at current density of 2 mA cm−2 with enhanced cycling stability (91% capacity retention over 1000 cycles) when tested as supercapacitor electrode in a three-electrode system. Using active carbon as the negative electrode and the C3N4 decorated Ni2P2O7 nanoarrays as positive electrode, the supercapacitor delivered high pseudocapacitance of 862 mF cm−2 at current density of 2 mA cm−2 with favorable cycling stability. Its excellent performance was attributed to the C3N4 nanosheets covering the Ni2P2O7 and Ni foam to prevent the Ni2P2O7 nanoarrays from peeling during charging and discharging. The composite materials exhibited high pseudocapacitance and long cycling stability. To prepare efficient supercapacitors with excellent cycling, Matheswalan et al.283 prepared NiCoP2O7/g-C3N4 (NP3) via the solvothermal method, in which NiCoP2O7 NPs were anchored on g-C3N4. The as-prepared NiCoP2O7/g-C3N4 composite material delivered a specific capacitance of 342 F g−1 at a scan rate of 5 mV s−1. The electrode exhibited excellent cycling stability (with 100% capacitance retention over 5000 cycles) in supercapacitors. Its excellent electrochemical performance and cycling stability were attributed to the interaction at the interface, which facilitated electron ion transport. g-C3N4 has attracted extensive research due to its unique 2D π plane structure, high nitrogen content, excellent mechanical strength, flexibility, thermal stability, and chemical stability. However, there has been relatively little research on supercapacitors. Li and colleagues284 reported that an integrated electrode of NiCo2O4 nanoneedles@g-C3N4 ultrathin film core-shell nanostructures/CC (NiCo2O4 NNs@g-C3N4/CC) was obtained, and the as-obtained integrated electrode displayed excellent electrochemical performance, exhibiting an areal capacitance of 2.83 F cm−2 at current density of 1 mA cm−2 as well as high cycling stability (5.88% loss after 10,000 cycles) and outstanding rate capability (1.13 F cm−2 at current density of 15 mA cm−2). PEDOT@graphite-phase C3N4 (PEDOT:PSS@g-C3N4) composite materials were prepared via a simple direct mixing process by Guo and colleagues.285 The as-obtained composite materials delivered a good specific capacitance of 277 F g−1 at 1 A g−1 in 1 M H2SO4. The PEDOT:PSS@g-C3N4 exhibited excellent energy density (10.2 Wh kg−1 at a power density of 4245 W kg−1) and cycling stability (with capacitance retention of 94.2% after 5000 charge/discharge cycles). Carbon and CuO nanospheres anchored on g-C3N4 nanosheets (C/CuO@g-C3N4) as supercapacitor electrode, which was self-assembled via a one-step co-pyrolysis decomposition method, was synthesized by Vattikuti et al.286 The C/CuO@g-C3N4 hybrid materials delivered 247.2 F g−1 in 0.5 M NaOH at current density of 1 A g−1 and excellent cycling stability (with more than 92.1% of the capacitance retained after 6000 cycles). Tao and colleagues287 reported that novel flower-like PANI/g-C3N4 hybrid materials were fabricated via an in situ oxidative polymerization method. The as-obtained PANI/g-C3N4 exhibited high performance (capacitance of 584.3 F g−1 at current density of 1 A g−1) and cycling stability (capacitance retention of 81.91% after 1000 cycles at current density of 1 A g−1). Nitrogen-rich, few-layer-thick, holey g-C3N4 nanosheets, which were intended for charge-storage supercapacitor (energy-storage) applications, were synthetized via a simple, novel, direct thermal polymerization method by Antil et al.,288 as shown in Figure 20A–G. The obtained composite material exhibited electrochemical double layer capacitor behavior in alkaline electrolyte, which delivered high specific capacitance (275 F g−1), energy density (30 Wh kg−1), power density (6651 W kg−1), and cycling stability (with >98% capacitance efficiency retained up to 10,000 measured cycles). Alagiri and colleagues289 fabricated ZnFe2O4/g-C3N4 hybrid material via a facile sol–gel method. The as-prepared ZnFe2O4/g-C3N4 exhibited high electrochemical supercapacitance performance, with high current density (103 F g−1 at current density of 10 A g−1) and excellent capacitance retention (with 94% of capacitance retained at current density of 10 A g−1 after 500 cycles). Novel sandwich-like g-C3N4/PPy composite materials, in which PPy was anchored onto the surfaces of layered g-C3N4 nanosheets, were synthesized via the chemical oxidation method by Dong et al.,290 as shown in Figure 20H–Q,S,T. The as-prepared g-C3N4/PPy NC delivered a large specific surface area (281 m2 g−1) and high specific capacitance (471 F g−1 at current density of 1 A g−1), good cycling stability (with >80% of its original capacitance retained with cycling up to 1000 times at 20 A g−1), as well as high rate capability characteristics. The excellent electrochemical performance was attributed to the following reasons: the unique sandwich structure that facilitates the diffusion of ions and electrons and the composite's ability to buffer the volume changes during the charging and discharging processes, improving cycling performance. Guo and colleagues291 fabricated SnS2/g-C3N4 composite materials via the hydrothermal route. The as-obtained SnS2/g-C3N4 showed good specific capacitance (552 F g−1 at 0.5 A g−1) and delivered excellent cycling stability (95.8% capacitance retention after 15,000 cycles at current density of 10 A g−1). Its high-quality electrochemical performance was due to its unique structure, while the high N content in g-C3N4 was beneficial for improving the conductivity of the material. The SnS2 was also loaded on g-C3N4, avoiding SnS2 agglomeration and improving cycle life performance. Liu et al.292 reported that novel 3D manganese sulfide (MnS)/Co9S8 (MCS) microflowers composite, which was loaded on Ni foam, was prepared by the hydrothermal method. MCS had a unique serrated edge structure, and it showed excellent specific capacity (1070 C g−1 at current density of 1 A g−1) and cycling stability (86% capacitance retention after 1000 cycles).
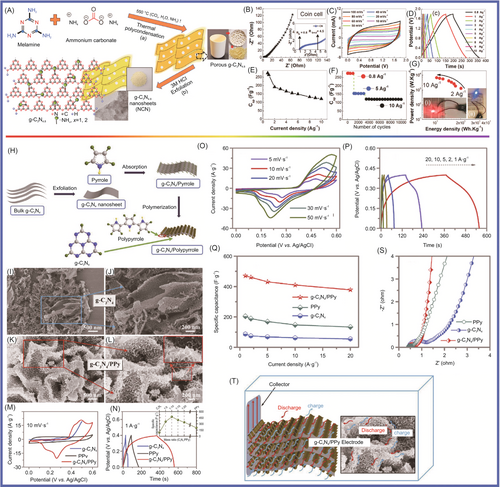
Novel polycarbazole (PCz)-g-C3N4 hybrid supercapacitor electrode material, which exhibited the high capacitance of 199 F g−1 at current density of 1 A g−1, was synthesized via a chemical oxidative polymerization method by Praveena et al.293 The as-prepared PCz-g-C3N4 was applied in supercapacitors, which delivered stable cycling (no significant variation after 1000 cycles). Tao and colleagues294 prepared a new active electrode material for supercapacitors via in situ oxidation polymerization, in which and were anchored in polyaniline (PANI)/g-C3N4 (Ag/PANI/g-C3N4). The as-obtained Ag/PANI/g-C3N4 exhibited excellent specific capacity of 797.8 F g−1 at 1 A g−1 in 1.0 M H2SO4 electrolyte and good cycling stability (capacitance retention of 84.43% after 1000 cycles at current density of 1 A g−1). Xu et al.295 reported that a novel flower-like g-C3N4/MoS2 heterojunction composite was synthesized via a facile one-step hydrothermal process. The as-synthetized g-C3N4/MoS2 delivered high specific capacitance (532.7 F g−1 at 1 A g−1) and excellent cycling stability (88.6% capacitance retention at 1 A g−1 after 1000 cycles). The improvement in electrochemical performance was attributed to the combination of g-C3N4 and the flower-like MoS2, which provided a good charge transfer field and electrolyte diffusion channels, and effectively prevented corruption and aggregation during charging/discharging processes. Wang et al.296 reported that AgNPs@g-C3N4 composite materials were prepared by an ultrasonic-assisted self-assembly method. The as-obtained AgNPs@g-C3N4 materials exhibited specific capacitance of 28.8 F g−1 at 1.0 A g−1. The capacitance of the composite material was 14.1 F g−1 at current density of 5.0 A g−1. The AgNPs@g-C3N4 composite material exhibited excellent performance, which was attributed to the following reasons: the composite materials had a high specific surface area, while Ag NPs improved the conductivity of the materials and promoted charge transfer. Sun et al.297 designed and synthesized MnO2/g-C3N4@PPy ternary NC (MnO2/g-C3N4@PPy NC) via facile soft chemical routes. The as-prepared MnO2/g-C3N4@PPy NC delivered highly enhanced supercapacitor performance (specific capacitance of 274 F g−1 at 2 A g−1) and cycling stability (95% capacitance retention after 1000 cycles). Ragupathi et al.298 fabricated g-C3N4 doped with MnS (g-C3N4-doped MnS) NPs by the sol–gel technique. The structural and supercapacitive behavior of as-prepared spherical MnS-doped g-C3N4 promoted electron transfer, buffered volume changes during charging and discharging, and improved stability, delivering high specific capacitance (463.32 F g−1) and specific capacitance retention (98.6% retained after 2000 cycles). Tang et al.299 prepared 3D g-C3N4/PEDOT:PSS as an electrode material for supercapacitors, which increased the specific surface area and showed good electrochemical performance. The 3D g-C3N4/PEDOT:PSS achieved specific capacitance of 202 F g−1 at a scanning speed of 5 mV s−1, good cycling stability (83.5% of initial capacitance maintained after 5000 cycles), and flexibility. PPy, as a conductive polymer, has a wide range of applications in energy storage devices. Li et al.300 reported that they had fabricated tubular g-C3N4 (TGCN) for application in supercapacitor electrodes. To further improve the performance of the supercapacitors, the core-shell structure of a nanofiber-reinforced cell (NFC)/ppy/TGCN film, where ppy is PPy, was prepared through polymerization and vacuum induction. The prepared NFC/ppy/TGCN exhibited excellent performance, with areal capacitance of 2.53 F cm−2 at 5 mA cm−2, which was mainly attributed to the unique structure of NFC/ppy/TGCN (improved electron transfer efficiency and good wettability). 2D g-C3N4 exhibits unique surface properties due to its lone pair electrons, which facilitate electrolyte infiltration. Exfoliated g-C3N4 is prone to agglomeration during the charging/discharging process, however, which limits its application in capacitors. A 2D nanohybrid structure of g-C3N4/MoS2 was fabricated hydrothermally by Kavil et al.301 The prepared g-C3N4/MoS2, which employed both faradaic and nonfaradaic processes for energy storage, exhibited excellent specific capacitance (45.5 F g−1), which was superior to the specific capacitances of g-C3N4 (10 F g−1) and MoS2 (14 F g−1) alone. Li et al.302 synthesized NFC/g-C3N4 by a vacuum-induced self-assembly process. The as-prepared NFC/g-C3N4, as supercapacitor electrode, delivered good specific capacitance (51 F g−1 at current density of 1 mA cm−2 and 46 F g−1 at current density of 1 mA cm−2), which was different from g-C3N4 alone. The excellent performance was attributed to the following reasons: the high nitrogen content of g-C3N4 facilitated electrolyte infiltration and improved transport performance, while the large specific surface area facilitated charge transfer. Lin et al.303 reported carbon-coated g-C3N4 nanotubes produced through urea thermal polymerization and glucose carbonization. When the mass ratio of urea to glucose was 30:1, the as-prepared carbon/g-C3N4 hybrid (TCN-200) was successfully prepared by recrystallization in water, drying, and calcination at 550°C for 2 h. The as-prepared TCN-200 delivered high specific capacitance (241.6 F g−1 at 1 A g−1), which was about 1.9 times that of bulk g-C3N4 (BCN) (124 F g−1 at 1 A g−1). Urea and glucose combined through hydrogen bonds to form a layered structure, and the carbon dots generated by glucose carbonization prevented the aggregation of g-C3N4. Its excellent performance was attributed to its unique structure. Energy density has limited the development of supercapacitors. To improve energy density, commonly used research methods mainly relied on doping heteroatoms or forming composites with other active materials. Bai et al.304 prepared Co3O4/g-C3N4 hybrid material, forming p–n junctions and exhibiting excellent capacity in supercapacitor devices, as shown in Figure 21A–O. The as-synthesized Co3O4/g-C3N4 hybrid material was made into shell-free supercapacitor devices (CoCN//CoCN ASSD, where ASSD stands for antisymmetric supercapacitor device). The energy density increased from 7.5 to 12.9 Wh kg−1 at a power density of 16.0 kW kg−1. The lattice distortion of Co3O4, the reduction of g-C3N4 defects, and the photogenerated charge separation promoted by the Co3O4/g-C3N4 p–n junctions all contributed to improving the electrochemical storage performance.
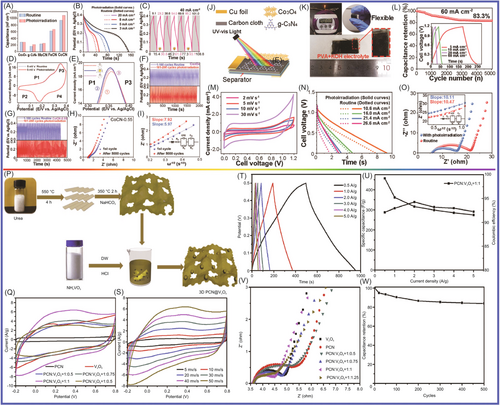
To improve the performance of supercapacitors, Nabi et al.306 prepared TiS2, g-C3N4, and TiS2/g-C3N4 composite hybrid material by the hydrothermal method. The as-prepared TiS2/g-C3N4 hybrid material delivered higher specific capacitance (546 F g−1) and excellent stability (retaining more than 87% of the capacitance after 2500 cycles), compared with TiS2 (292 F g−1) and g-C3N4 (200–300 F g−1). This excellent performance was attributed to smaller particle size, additional active sites, and a strong synergetic effect. Murugan et al.307 prepared g-C3N4/BiVO4 by the hydrothermal method, which was applied in a high-performance symmetric supercapattery device as supercapacitor electrode. 6 wt% g-C3N4 was introduced into as-synthetized g-C3N4/BiVO4, which delivered high specific capacity (2171 C g−1 at 2 A g−1). Its excellent performance was attributed to its battery-type Faradic redox reaction (noncapacitive) and the synergistic effect of both capacitive and noncapacitive contributions by g-C3N4 and BiVO4. Symmetric supercapacitor devices were fabricated from g-C3N4/BiVO4 hybrid material, which showed a maximum potential of 2 V and good electrochemical stability (130% with Coulombic efficiency of 98.8% even after 20,000 cycles at a high current density of 20 A g−1). The power density and energy were 16.2 and 61 Wh kg−1, respectively. g-C3N4 nanosheets were fabricated via a chemical oxidation method and a thermal oxidation method by Zhang et al.308 The application of g-C3N4, oxidized g-C3N4, and thermally oxidized g-C3N4 were studied in supercapacitors, demonstrating different electrochemical performance. Compared with g-C3N4 (127.7 F g−1 at current density of 0.5 A g−1) and oxidized g-C3N4 (after treatment with 12 M sulfuric acid [specific capacity of 133.6 F g−1 at current density of 0.5 A g−1]), thermally oxidized g-C3N4, which was exposed to thermal oxidation at 580°C, exhibited the optimal electrochemical performance (170.1 A g−1 at current density of 0.5 A g−1) and cycling stability (with a capacity retention of 95.9% after 1000 cycles in 2 M KOH). As shown in Figure 21P,Q,S–W, Zhou et al.305 reported that a composite of 3D porous-structured g-C3N4 and vanadium pentoxide (V2O5) (3D PCN@V2O5) had been prepared via the solvothermal method. The 3D PCN@V2O5 exhibited high specific capacity of 457 F g−1 at current density of 0.5 A g−1 and excellent cycling stability (approximately 84% capacity retention after 500 cycles). This excellent performance was attributed to the following reasons: g-C3N4 was low cost and had excellent chemical stability, which was conducive to carrier extraction and transport in the vertical direction. Sun et al.309 fabricated a composite of thermally oxidized carbon with self-doped g-C3N4/nickel sulfide particle (TC-g-C3N4/NiS), in which TC-g-C3N4 was synthesized via solvothermal treatment of melamine in ethanol and thermal oxidation peeling, while NiS particles were attached to the surface and inside the TC-g-C3N4 layers via the hydrothermal method, as shown in Figure 22. g-C3N4 enhanced the charge transfer through self-doping and created the conditions for NiS introduction through etching. NiS particles were adsorbed on g-C3N4 to avoid aggregation. The as-prepared TC-g-C3N4/NiS exhibited excellent specific capacity of 1162 F g−1 at 1 A g−1 and remained stable at 82% after 8000 cycles. The assembled supercapacitor structure was TC-g-C3N4/NiS/AC, exhibiting high energy density of 27 Wh kg−1 and excellent cycling performance (with a capacity retention rate of 87.9% after 8000 cycles). Increasing the specific surface area of electrode materials has been one of the most effective ways to improve the performance of capacitors. Qu et al.310 prepared graphene/g-C3N4 for application in supercapacitors. The introduction of g-C3N4 nanosheets was beneficial for increasing charge storage. The interaction between C and N in g-C3N4 and O in graphite oxide could effectively avoid agglomeration of graphite oxide and increase the specific capacity. The as-prepared materials delivered excellent performance (1500 mF cm−2), cycling stability (95% of initial capacitance after 5000 cycles), and maximum energy density of 0.075 mWh cm−2. Chung and colleagues311 prepared NiO/g-C3N4 (NC) hybrid material by the hydrothermal method, using melamine and nickel hydroxide as precursors. The ratio of Ni(OH)2 to melamine and the heat treatment temperature were studied to obtain the optimal preparation conditions. As an electrode material prepared under optimized conditions, it exhibited excellent specific capacity (49.6 Wh kg−1 at a power density of 1064.2 W kg−1 at 1 A g−1) in symmetric supercapacitors. Lu and Chen312 prepared CNTs/g-C3N4 nanosized hybrid material with a large specific surface area, porous structure, and high conductivity, and applied it in supercapacitors. In the charge/discharge process, CNTs/g-C3N4 hybrid material with high porosity and large specific surface area provided a storage layer for ions and promoted carrier transport. The as-synthetized CNTs/g-C3N4 showed excellent specific capacity (148 F g−1 at 1 A g−1), cycling performance (capacitance retention of 93% even after 10,000 cycles at 1 A g−1 under 0.8 V), and high rate capability (over a wide current density range from 1 to 10 A g−1). Its excellent properties were attributed to the fact that the material had a large specific surface area to increase the specific capacity and also had many nitrogen-active sites to promote charge transfer. Parida and colleagues313 prepared NiCo2O4/O-g-C3N4 (NCO/O-g-CN) NCs through multistep synthesis processes (hydrothermal and precipitation methods). The introduction of O-g-C3N4 was beneficial for improving the superelectric performance. The as-prepared NCO/O-g-CN exhibited excellent specific capacity of 438.1 C g−1 at current density of 1 A g−1 and excellent cycling stability (with a retention rate after 10,000 cycles of 93.15% at current density of 4 A g−1). The excellent performance of NCO/O-g-CN hybrid material was attributed to the increase in the specific capacity of a single material through synergistic action. To meet the continuous demand for supercapacitors, it is crucial to prepare high-energy, high-power-density, stable, and low-cost electronic materials. Allam and colleagues314 prepared g-C3N4/Bio-Cx mesoporous materials as electrode materials. The prepared material exhibited excellent specific capacity of 300 F g−1 at current density of 1 A g−1. In the device (GCN/Bio-Cx//MPNDC), GCN/Bio-Cx composite material was used as the positive electrode, and mesoporous nitrogen-doped carbon (MPNDC) was used as the negative electrode to assemble a supercapacitor that had an energy density of 53.72 Wh kg−1 and a power density of 900 W kg−1. After 13,000 charges/discharges, the Coulombic efficiency of the GCN/Bio-Cx//MPNDC device was maintained at 100%. This excellent performance was attributed to the following advantages: the unique 3D/2D structure, the large surface area, and the huge number of available active sites. Designing and preparing special surface-functionalized materials to improve device performance has become one of the main research directions in this field. In terms of supercapacitors, bismuth vanadate anchored CNTs served as anode, amorphous Ni-Zn-B/g-C3N4 nanosheet network structures served as cathode, and dopants provided nitrogen dopants and defect sites in the BiVO4/NC produced by Khalafallah et al.315 The Ni-Zn-B/g-C3N4/BiVO4/NC device exhibited excellent high energy density (78.4 Wh kg−1 at a power density of 907.7 W kg−1, at operating voltage of 1.6 V) and excellent cycling stability (retention rate of 85.3% after 8000 cycles). The Ni-Zn-B/g-C3N4 3D heterojunctions, as the cathode material for the devices, had a unique structure and a large number of electrochemical active sites, which was conducive to improving the large specific capacity. The nanosheet network structure had large pores, promoting charge transfer and buffering structural changes. In BiVO4/NC, Bi and V ions with different valences improved the electrochemical performance, and the presence of N improved the electronic conductivity and structural stability. The synergistic effect facilitated ion and carrier transport, ultimately achieving improved device performance.
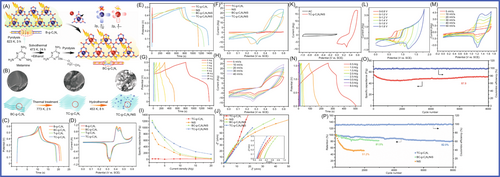
Co3O4 has attracted widespread attention from researchers as an electrode material for supercapacitors. Its low ion diffusion and conductivity, however, have limited its widespread application. To improve the low electron transfer rate of Co3O4, Zhang et al.316 prepared Co3O4/g-C3N4 composite materials, where Co3O4 was uniformly dispersed on the surface of g-C3N4. The as-synthetized Co3O4/g-C3N4 exhibited high specific capacitance of 1071 F g−1 at 1 A g−1, high energy density of 20.4 Wh kg−1 at a power density of 0.8 kW kg−1, and good cycling stability (80% capacitance retention after 4000 cycles at 10 A g−1). The introduction of g-C3N4 was beneficial for improving the electron transfer performance of Co3O4 nanosheets. Compared with other Co3O4 composite materials, the as-prepared Co3O4/g-C3N4 composites had excellent electrochemical stability, high specific capacity, low internal resistance, and other advantages. Zhang and colleagues106 used electrospinning technology and the calcination route to prepare nanofibers. Thiourea was used as a source of nitrogen content, and the thiourea decomposed to form g-C3N4 carbon nanofibers and g-C3N4 composite materials with a nitrogen content of 19.78 at%. Among them, the total amount of pyrrolic nitrogen and pyridinic nitrogen was as high as 59.51%. The as-prepared C3N4@NCNF-600 as the anode of PIHCs exhibited excellent performance (energy density of 62 W kg−1 and power density of 2102 W kg−1) and good cycling stability. Ravi and colleagues317 prepared uniformly graded mesoporous 3D sea-urchin-like Bi2S3 that formed a composite material with 2D C3N4. The as-synthetized 3D-Bi2S3@2D-g-C3N4 displayed high capacity of 41.53 μAh cm−2 at 1 mA cm−2, with good rate performance of 62.77% and good cycling performance (retention of 94.86% after 5000 cycles). The improvement in device specific capacity, rate performance, and stability was attributed to the mesoporous structure of the material, high specific surface area, high carrier transport efficiency, synergistic effects, and low interface resistance. The rapid and effective transformation of lanthanide oxides and hydroxides between oxidized states has attracted extensive research from researchers. Vivek et al.318 successfully anchored a novel Nd(OH)3 nanocoil onto 2D g-C3N4 nanosheets through a surface precipitation method. The characterizations showed that Nd(OH)3 was successfully anchored on g-C3N4, which facilitated carrier diffusion at the electrode material/electrolyte interface. Compared with Nd(OH)3 (247.52 F g−1) and g-C3N4 (111.60 F g−1), Nd(OH)3/g-C3N4 had a better specific capacity (426.40 F g−2 at current density of 1 A g−1) in 3 M LiOH. Nd(OH)3/g-C3N4 showed excellent cycling stability, maintaining 88.3% capacitance after 3000 cycles at 3 A g−1. The as-prepared Nd(OH)3/g-C3N4 material, due to its adjustable structure and high specific area, was conducive to electrolyte diffusion and improved charge transfer. Nd(OH)3/g-C3N4 had a large number of active sites, which were conducive to carrier migration at the interface. To develop high-performance supercapacitor devices, Joo and colleagues319 designed and prepared carboxyl functionalized (f)-g-C3N4 and a carbon nanofibers/TNS composite material through a simple impregnation method. The f-g-C3N4/CNF-TiO2 and g-C3N4/CNF-TiO2 electrode materials were obtained. The f-g-C3N4/f-CNF-TNS hybrid materials showed specific capacitance of 350 F g−1 at 5 mV s−1. The prepared material had excellent cycling stability (with a specific capacity of 89.2% after 2000 cycles). Composite materials exhibited excellent conductivity, surface functionalization, and chemical interfaces. The composite had a high specific surface area and improved the active sites of the reaction surface. Baruah et al.320 prepared graphitic phase carbon nitride and graphite oxide via self-assembled van der Waals heterojunctions of protonation, using the active sites before the interface. PPy nanotubes were introduced through in situ polymerization into rGO pg-CN, and rGO-pg-CN/PPyNT NC materials were obtained with a specific surface area of 158 m2 g−1 and an average pore size of 3.22 nm. The introduction of PPyNTs could inhibit the aggregation of the rGO-pg-CN nanosheets. The preparation of ternary rGO-pg-CN/PPyNT/ITO electrodes, where ITO is indium tin oxide, showed a capacitance of 803 F g−1 at current density of 0.5 A g−1, specific energy of 188.49 W kg−1, and specific power of 324.83 W kg−1 at current density of 0.5 A g−1. The as-synthetized rGO-pg-CN/PPyNT/ITO electrode delivered excellent cycling performance (with a rate capacity of 61% and cycling stability at 82% after 5000 cycles). S-doped g-C3N4/cobalt disulfide (S-g-C3N4/CoS2) material was prepared through in situ pyrolysis and application in high-performance hybrid asymmetric supercapacitors by Vinoth et al.321 The synthesized S-g-C3N4/CoS2 hybrid material showed a high specific capacity of 180 C g−1 and current density of 1 A g−1. At extremely high current density of 30 A g−1, the capacitance under electrochemical high stability cycling was 89% after 100,000 cycles, and the Coulombic efficiency was 99.6%. In aqueous electrolytes, the energy was as high as 32 Wh kg−1, and the power density was 19.8 kW kg−1. CoS2 was anchored in the S-g-C3N4 framework, which shortened the carrier transmission distance and enhanced the interface effect, improving the device performance and structural stability. MoS2 has been widely studied as an electrode material for supercapacitors. The conductivity of pure MoS2 was investigated, but this material was found to be prone to agglomeration, hindering its electrochemical performance and greatly limiting its practical application. Co-doped MoS2 was prepared in a composite with g-C3N4, which improved its electrochemical performance. Lu et al.322 prepared Mo0.7Co0.3S2 (MCS)/g-C3N4 via the solvothermal method. The MCS/g-C3N4 NC exhibited a highly crystalline hexagonal structure, with MCS anchored on the surface of g-C3N4. The MCS/g-C3N4 NC material showed excellent electrochemical performance, especially the MCS/CN-2 electrode (with a mass ratio of MCS:g-C3N4 of 9:1) exhibiting the best electrochemical performance. The initial specific capacitance of MCS/CN-2 was 929.5 F g−1. The specific capacitance of MCS/CN-2 was 907.0 F g−1 after 3000 cycles, so the capacitance retention of the MCS/CN-2 sample was 97%. Ranjithkumar et al.323 prepared g-C3N4/TiO2 hybrid material by thermal diffusion method and studied the influence of g-C3N4 loading on the physicochemical properties of the hybrid material. The surface of TiO2 was coated with g-C3N4. As the loading amount increased, the structure of g-C3N4/TiO2 became blocky. The influence of different loadings on the chemical properties was studied, and 33% of the g-C3N4/TiO2 hybrid material showed the optimal specific capacitance. The synthesized g-C3N4/TiO2 hybrid material had a specific capacity of 864.5 F g−1 at 1 mV s−1 and excellent cycling stability. Ji et al.324 prepared co-doped g-C3N4 materials (Co-CN) through high-temperature thermal polymerization. The introduction of Co ions improved the material interfaces and enhanced electrochemical performance. As the calcination temperature increased, the Co-CN sample peeled off into thin nanosheets, and the introduction of Co was beneficial for the nanosheets to be rolled into nanotubes. The Co-g-C3N4 composite nanotubes exhibited high charge/discharge capacity (at a potential of 249 mV at current density of 10 mA cm−2). Ghosh et al.325 prepared NiCo2C2O4 by a simple coprecipitation method with a specific capacity of 1009 F g−1 and a current density of 1 A g−1. The introduction of g-C3N4 led to the formation of nanoporous NiCo2C2O4/g-C3N4 composite materials, where NiCo2C2O4 flakes were uniformly loaded on g-C3N4. The specific capacity of the synthesized nanoporous NiCo2C2O4/g-C3N4 composite material was 1263 F g−1 (158 mAh g−1) at 1 A g−1. The nanoporous material delivered a high specific energy of 226.36 W kg−1 and a specific power of 35.54 Wh kg−1. The performance improvement was attributed to the synergistic effect between Ni and Co ions. Arora et al.326 synthesized g-C3N4 and PPy composite materials as energy storage electrode materials. A series of g-C3N4/PPy (pcn) NCs (with unchanged pyrrole content and varying g-C3N4 content) were studied, in which oxidized pyrrole was prepared through in situ polymerization. The optimal specific capacity of 0.4 pcn was 555 F g−1 with a scanning rate of 10 mV s−1, an energy density of 86 Wh kg−1, and a power density of 300 W kg−1. Qiu et al.327 designed and prepared g-C3N4-modified NiCo layered double hydroxide in the form of self-growing nanosheet arrays (CN-LDH), which were prepared using microwave-assisted methods. The as-synthetized CN-LDH showed a high specific capacity of 1936.36 F g−1 at 1 A g−1, and it still maintained 87.79% of its initial capacity after 6000 charge/discharge cycles. The energy density reached as high as 50.63 Wh kg−1 at 0.80 kW kg−1. The g-C3N4 was introduced into the LDH, which effectively improved the device performance. The formation of an internal electric field at the p–n heterojunction interfaces facilitated charge transfer, which has become one of the means used to improve electrochemical storage performance. Wang et al.328 prepared a 3D sea-urchin-like CoNixSy/g-C3N4 (3D/2D) heterojunctions, in which g-C3N4 nanosheets were coated with sea-urchin-like metal sulfide microspheres. The prepared CoNixSy/g-C3N4 exhibited excellent specific capacity of 1029 C g−1. The asymmetric supercapacitor structure that was assembled was CoNixSy/g-C3N4/AC, with an energy density of 71.9 Wh kg−1 at 0.23 kW kg−1 and excellent cycling stability (maintaining a capacity retention rate of 72.2% after 5000 cycles). The presence of p–n heterojunctions significantly improved the performance in supercapacitors. Polat and Mashrah329 directly prepared g-C3N4/MgFe2O4 (g-CN-MFO) composite on the surface of nickel foam by the hydrothermal method, forming sponge-structured g-CN-MFO electrode. The g-CN-MFO presented an energy density of 13.3 mW cm−2 and a power density of 200 mW cm−2. Liang et al.330 prepared g-C3N4@NiMoO4/CoMoO4 (gCN@NCM) via the hydrothermal method, forming a 3D structure. Among them, the NCM rods were hollow structures, and g-C3N4 was inserted into the NCM rods to provide channels for carrier transport. The as-synthetized gCN@NCM showed good specific capacity of 641.5 F g−1 at 1 A g−1 and good cycling stability (maintaining 110% of its initial specific capacitance after 10,000 cycles). Ensafi et al.331 reported that they had anchored Ni2CoS4 onto the surface of g-C3N4 nanosheets through hydrothermal method. The as-prepared Ni2CoS4@g-C3N4 composite material exhibited high specific capacity (246 mAh g−1 or 2210 F g−1 at current density of 1 A g−1), excellent rate performance (about 86% retention at 40 A g−1), and high cycling stability (retained charge storage capacity of 85% after 4000 charge/discharge cycles). Subhash and colleagues332 prepared spherical NiS/g-C3N4 hybrid material by a one-step hydrothermal method. In the prepared hybrid material, NiS/g-C3N4, g-C3N4 was in the form of nanosheets and NiS was in the form of spherical nanostructures. The as-prepared NiS/g-C3N4 hybrid material exhibited excellent specific capacity of 2661.25 C g−1 at 1 A g−1. The assembled device structure was NiS/g-C3N4/AC with an optimal specific capacity of 181.8 C g−1 at 1 A g−1, which exhibited excellent cycling stability (95% capacity retention after 10,000 cycles), an energy density of 53.09 Wh kg−1, and a power density of 31537.5 W kg−1. Ma et al.333 designed and prepared g-C3N4/rGO/Ni(OH)2 through a microwave method. The synthetized g-C3N4-rGO nanosheets were successfully inserted into Ni(OH)2, and the introduction of the g-C3N4-rGO nanosheets increased the specific surface area of the material, improved carrier transport, and buffered volume changes during charge/discharge. The prepared g-C3N4/rGO/Ni(OH)2 exhibited excellent specific capacity (specific capacitance of 1887.4 F g−1 at 1 A g−1) and cycling stability (91.4% capacitance retention after 2000 cycles). Panicker et al.334 reported that they had fabricated porous C-C3N4/rGO@NCS (pCRNCS) composite materials via the solvothermal method, subsequently followed by calcination and acid treatment. High-specific-surface-area rGO and porous carbon could self-repair the defect states in g-C3N4 nanosheets, promoting the nucleation and growth of NCS. Compared with g-C3N4, carbon self-healing g-C3N4 (CCN) exhibited excellent electronic conductivity. This was due to the replaced C atom in the g-C3N4 structure, which extended the delocalized π electronic structure. After acid treatment, the larger planar structure of CNN decomposed into small fragments, increasing the edge N and O functional groups. The thus-constructed pCRNCS/AC asymmetric supercapacitor showed significant electrochemical performance (specific capacitance of 211 F g−1 at current density of 1 A g−1), outstanding cycling stability (capacitance retention of 93.6% after 6000 cycles), and power density (optimum energy density of 66 Wh kg−1 at a power density of 751 W kg−1). The as-fabricated Ag-NiAl2O4@g-C3N4, which exhibited specific capacity of 768 F g−1, outstanding cycling stability (94.27% capacitance retention after 6000 cycles), power density of 187.28 W kg−1, and energy density of 27 W kg−1, showed better performance with low charge transfer resistance (0.51 Ω), as reported by Irshad and colleagues.335 Silver doping and g-C3N4 sheets, which provided more charge storage sites and increased specific capacitance, could effectively increase the conductivity and specific surface area of the composite material. El-Sabban et al.336 prepared porous sulfur-doped g-C3N4/Bi2S3 (Sg-C3N4/BiNS3) composite materials using a template-free method and applied them in hybrid asymmetric supercapacitors. The porous Bi2S3/Sg-C3N4 composite material had specific capacity of 670 F g−1 (235 C g−1) at 1 A g−1 and excellent cycling stability (retained 80% of its initial capacity after 3000 cycles). The specific energy and specific power were 455 W kg−1 and 6.2 Wh kg−1, respectively, at current density of 0.8 A g−1. Zhou et al.337 reported that NCS nanomaterials@C3N4 (NCS@C3N4) composite hybrid materials were fabricated via the solvothermal method. The NCS@C3N4 displayed specific capacity of 890.4 C g−1 at current density of 1 A g−1 and showed good cycling stability. NCS was evenly distributed on the C3N4. The introduction of NCS increased the C3N4 layer spacing and buffered structure changes, which was conducive to the exposure of active sites and carrier transfer.
2.6 g-C3N4 applied in zinc-ion battery
Zinc metal has advantages such as low cost, high safety, high theoretical specific capacity (820 mAh g−1), and low redox potential (−0.76 V vs. standard hydrogen electrode).338-340 However, when it is applied to the negative electrode of water-based zinc-ion batteries, uneven zinc deposition leads to the formation and growth of zinc dendrites, piercing the separator and causing battery short circuits.341-344 Zinc dendrites have mechanical rigidity and structural nonuniformity, which can easily detach from the zinc negative electrode to form “dead zinc,” leading to a decrease in active materials and a decline in battery capacity. The coating functionalizes the surface of zinc negative electrode to suppress zinc dendrites and related side reactions, improve the stability of zinc metal negative electrode, and thereby enhance the electrochemical performance of aqueous zinc-ion batteries.345-347
As a key component of zinc-ion batteries, the separator played an important role in regulating zinc-ion flux, thereby directly affecting the growth of zinc dendrites. Wu et al.348 reported for the first time using 2D g-C3N4 nanosheets to modify glass fiber membranes. The g-C3N4 layer could effectively avoid penetration and suppress self discharge of zinc-ion batteries. The g-C3N4/GF separator delivered excellent performance (280 mAh g−1 at 1 C after 400 cycles) in zinc-ion batteries. Zinc metal was considered one of the most promising electrode materials for aqueous zinc-ion batteries due to its high theoretical specific capacity, low cost, and environmental friendliness. The growth of crystal branched and sided reactions on the surface of zinc anodes result in low Coulombic efficiency and limited lifespan. Wang and colleagues349 reported a metal surface modification method to construct a stable and sturdy g-C3N4 (g-C3N4@Zn) on the surface of a metal zinc anode as a multifunctional protective layer. The synthetized g-C3N4@Zn was applied in zinc-ion batteries, which presented higher specific capacity of 153.1 mAh g−1 with almost 100% Coulombic efficiency after 1000 cycles. The excellent performance was attributed to the following factors: (1) the hydrogen evolution reaction and the formation of by-products can be effectively relieved by g-C3N4 coating layer; (2) the N-rich g-C3N4 layer exhibits inherent zincophilic properties, in which zinc ions can be bonded with N to form Zn-N bonds. Liu et al.350 achieved the goal of regulating the zinc interface based on 3D printing g-C3N4, which achieved uniform nucleation of zinc and inhibited dendrite growth. The prepared Zn/C3N4 was applied in zinc-ion batteries, providing lower energy barriers and more uniform charge distribution. The battery using 3D-printed g-C3N4 modulating coatings delivered excellent stability for 500 cycles with 94.1% at 1000 mA g−1. Xue and colleagues351 reported that the commercial cellulose separator was modified by 2D porous g-C3N4 nanosheets. g-C3N4-coated separator delivered a cycling stability of 750 cycles with an average Coulombic efficiency of 99.2%; the symmetric cell kept a stable voltage profile for over 300 h at 5 mA cm−2. The excellent performance was attributed to the following factors: (1) porous g-C3N4 coating redistributes the zinc-ion flux through the separator; (2) porous g-C3N4 coating improves the mechanical strength of the separator; (3) porous g-C3N4 modified separator enables a dendrite-free zinc anode. Manganese-based materials were widely developed for use as positive electrodes in zinc-ion batteries due to their advantages such as low cost, high safety, and simple preparation. However, severe volume changes and poor stability during the charging process limited their advancement and application. Gao and colleagues352 successfully prepared α-MnO2@g-C3N4 applied in zinc-ion batteries. The α-MnO2@g-C3N4 delivered specific capacity of 298 mAh g−1 at 0.1 A g−1. At 1 A g−1, α-MnO2@g-C3N4 still retained 100 mAh g−1 (83.4% retention after 5000 cycles) and excellent cycling stability. The α-MnO2@g-C3N4-based device presented high energy density (563 Wh kg−1) and power energy density (2170 W kg−1). Min and colleagues353 applied nano semiconductor g-C3N4 as a coating on zinc surface to achieve uniform dispersion of electric field and inhibition of zinc dendrite growth. The Zn symmetrical device could be stably cycled for 1000 h at 0.5 mA cm−2, with its overall areal capacity of 0.5 mAh cm−2, which was attributed to these benefits of the coating. They can be stably circulated for 500 h at a high current density of 5 mA cm−2, with its total areal capacity of 1 mAh cm−2.
2.7 g-C3N4 applied in zinc-air battery
Zinc-air batteries have advantages such as high theoretical energy density, abundant resources, environmental friendliness, and rechargeability, making them a promising new generation of energy conversion and storage technology.354-356 During the discharge process of zinc-air batteries, oxygen reduction reaction (ORR) occurs at the air cathode, and oxygen evolution reaction (OER) occurs during the charging process. However, the slow kinetics of ORR and OER results in lower power density and poor cycling stability.357-359 Platinum carbon and ruthenium/iridium compounds are efficient catalysts for oxygen reduction and evolution, respectively. However, their high cost and poor stability limit their large-scale application. In addition, these precious metals are often single-functional catalysts that require mixed use to achieve dual-functional catalytic activity.360-362 The development of efficient and stable dual functional nonprecious metal catalysts and air electrodes is the key to the practical application of rechargeable zinc-air batteries.
The g-C3N4@MWCNTs/Mn3O4 series catalysts were prepared by Li et al.363; they were used for ORR in zinc-air batteries. GMM35 (the loading of 35% Mn3O4) exhibited the best activity under alkaline conditions during the preparation of a half-cell test. Under alkaline conditions, GMM35 NCs exhibited lower peak power density (192.4 mW cm−2 at 229.1 mA cm−2) compared to commercial 20 wt% Pt/C catalysts. To reduce the production cost of rechargeable zinc-air batteries, it is necessary to integrate ORR and OER with high catalytic activity into a catalytic system. Wang and colleagues364 prepared Co@NHCC, which was direct pyrolysis of Co-MOFs (ZIF-67) polyhedron nanocrystals with the presence of dense silica NPs and additional high-temperature decomposable g-C3N4 (nitrogen source). The dense silica on the surface of ZIF-67 not only facilitates the incorporation of nitrogen atoms into the graphitized carbon skeleton but also effectively buffers the thin-walled collapse of ZIF-67 polyhedra and inhibits the growth of CNTs on the surface. When Co@NHCC-800 was used in zinc-air batteries as an electrode, it showed a much higher open-circuit voltage (1.490 V) and a higher discharge power density (248 mW cm−2) than Pt/C catalyst. The hybrid material composed of transition metal compounds and nitrogen-doped carbon carriers exhibits excellent catalytic performance. It is mainly attributed to the synergistic effect between metal sites and N dopants. However, the mechanism and synergistic effects of multimetal systems have been rarely studied. Deng and colleagues335 prepared NCS/graphite nitride carbon/CNT (NCS@g-C3N4-CNT) hybrid materials. There was a large amount of pyridine nitrogen present in g-C3N4. It showed that the bimetallic Ni/coactive sites transfer to g-C3N4, and the synergistic effect with the coupled CNT could promote reversible electrocatalysis. The optimized NCS@g-C3N4-CNT mixture, which presented a high specific discharge capacity of 480.7 mAh g−1 in all-solid-state zinc-air batteries, exhibited excellent dual functional performance in catalytic oxygen reduction/release reactions, which was very effective for low potential, high efficiency, and long lifespan practical zinc-air battery packs, surpassing physical mixing equivalents and state-of-the-art precious metal catalysts. Nitrogen-doped carbon materials are a promising low-cost catalyst for redox reactions. Wang and colleagues365 prepared porous nitrogen-doped carbon materials (porous N–C materials), which were prepared by using g-C3N4 embedded in glucose-derived carbon as a template and N source. When the mass ratio of glucose to g-C3N4 was 4:1 and the synthesis temperature was 900°C, it exhibited excellent catalytic performance. It is mainly attributed to the prepared materials having a highly specific surface area, high porosity, and a large amount of pyridine nitrogen and graphite nitrogen. When a small amount of Fe was doped in the N-C sample, its ORR performance could be greatly improved. When N-C and Fe-N-C were used as cathode catalysts in zinc-air batteries, the power densities were 88 and 100 mW cm-2, respectively. Yang and colleagues366 prepared efficient Co-modified N-doped carbon (NC/Co) through the pyrolysis method. When the Co doping content was 1 mmol, NC/Co-1 showed excellent performance (power density of 153 mW cm−2) in zinc-air batteries. It is attributed to the abundance of N in g-C3N4, which facilitates in situ Nitrogen doping and forms catalytic active sites for Co. Dey and colleagues367 prepared Fe-doped g-C3N4 (Fe-g-C3N4) via a one-step thermal polymerization method. When the Fe-g-C3N4 catalyst was used as a positive electrode material for zinc-air batteries, its peak power density (148 mW cm−2) was higher than that of Pt/C (133 mW cm−2). Fe could form active sites with N, which coordinated with Fe atoms. Fe was introduced in g-C3N4, which can effectively improve the specific surface area, and the graphitization and conductive carbon skeleton Fe-g-C3N4 provide electron transport pathways. Wang and colleagues368 synthesized Fe-doped bamboo-like N-doped CNTs, in which the N atoms in the g-C3N4 skeleton exhibited coordination effects, facilitating the formation of uniform CNTs, and Fe was anchored on the surface of g-C3N4, which was beneficial for reducing Fe aggregation. The Fe-FeNx@NCNTs presented in zinc-air batteries displayed a power density of 163 mW cm−2, higher than the reference device (that of the reference Pt/C device was 142 mW cm−2). Niu et al.369 obtained the optimal ratio of Fe-CNNs-0.7, Ni-CNNs-0.7, and Co-NNs-0.7 composite materials by adjusting the ratio of metal acetylacetonate to g-C3N4 precursor. The prepared composite hybrid materials exhibited excellent performance. In rechargeable zinc-air batteries, Co-CNNs-0.7, as a cathode material for zinc-air batteries, exhibited an excellent peak power density of 85.3 mW cm−2, and could maintain a specific capacity of 675.7 mAh g−1 after 1000 cycles. To obtain energy level matching and structural stability electrode materials, Lee and colleagues370 designed and prepared an n-type g-C3N4 and p-type copper-doped ZIF-67 composite material for use in zinc-air batteries. g-C3N4/CuZIF-67 exhibited excellent performance mainly due to its suitable bandgap, interleaved p–n heterojunction bandgap structure, and built-in electric field synergistic effect. It had a voltage gap of 0.81 V under 1-sun illumination with enhancing charge/discharge abilities via photo. The as-prepared g-C3N4/CuZIF-67 remained stable cycling with 60% round-trip efficiency under illumination over 1000 cycles for 336 h at 2 mA cm−2. Xu and colleagues371 designed a simple self-sacrificial template strategy to prepare porous-rich nitrogen-doped graphene co-anchored with Co single atoms and Co atomic clusters. During the preparation process, layered g-C3N4 was obtained through the pyrolysis of dicyandiamide, which was used as a sacrificial agent template to provide more anchoring points. To investigate the role of cobalt atoms in Co signle atoms (CoSA) catalysts/AC@NG, the cobalt salt was prepared by changing the initial amount of cobalt salt from 0 to 40 mg, denoted as NG, Co10SA/AC@NG, Co20SA/AC@NG, and Co40SA/AC@NG, respectively. The as-synthetized Co40SA/AC@NG exhibited optimal power density (221 mW cm−2) and cycle stability in zinc-air batteries. Lin and colleagues372 used rod-shaped S-doped g-C3N4 as a nitrogen-rich precursor and cobalt permeation and prepared Co@bCNT hybrid materials through a two-step method. N- and S-doped bamboo-like CNTs (bCNTs) were coated with Co NPs in Co@bCNTs, which combine with doped graphene to form an amorphous CoSx hybrid material. Assembled liquid and all-solid-state flexible batteries presented excellent performance (peak power densities of liquid and flexible batteries up to 190.3 and 106.2 mW cm−2). Zheng and colleagues373 designed and prepared core-shell S-g-C3N4@P123 material, in which S-doped g-C3N4 was prepared by pyrolysis of urea and thiourea. The as-prepared S-g-C3N4@P123 materials were used as catalysts in zinc-air batteries, which had shown excellent performance (the specific discharge capacity of zinc-air battery reached 684 mAh g−1 at 20 mA cm−2). Hu and colleagues374 prepared 2LaCo0.7Fe0.3O3/N-doped carbon via the sol–gel method, in which g-C3N4 nanosheets were carbon and nitrogen sources, and 2LaCo0.7Fe0.3O3 had excellent dispersion on N-doped carbon. The as-prepared 2LaCo0.7Fe0.3O3/N-doped carbon material displayed excellent cycling stability (over 24 h) and high peak power density (116 mW cm−2) in zinc-air batteries. Liu and colleagues375 designed and prepared Co-NCNT hybrid materials, in which 1D metal nitrogen-doped CNTs were prepared through 2D graphite-phase carbon nitride. At the end of Co-NCNT, there were Co@CoOx nanoblocks. After nitric acid etching, the Co-NCNT-N materials were obtained. The as-prepared Co-NCNT-N electrode material assembled into a zinc-air battery exhibited excellent performance (ultrahigh power density of 210 mW cm−2, small charge–discharge voltage gap of 0.80 V, and good stability of 130 h). Zhang and colleagues376 prepared N-doped carbon and encapsulated FeCo alloy materials through metal salt-doped G-g-C3N4 pyrolysis, and the prepared materials have active sites. The prepared materials exhibited excellent electrochemical performance (peak power density of 120 mW cm−2 with open-circuit voltage of 1.52 V) in zinc-air batteries.
Cobalt-based N-doped carbon has attracted extensive research as a multifunctional catalyst in metal N-doped carbon. Liu and colleagues377 designed and prepared an efficient bifunctional catalyst that had a Co-doped carbon skeleton by directly introducing Co ions during dopamine polymerization. Co-based MOFs polyhedra form Co-based NPs during the pyrolysis process. The prepared catalyst was applied in zinc-air batteries, which exhibited a stable discharge voltage of 1.1 V at the high current density of 10 mA cm−2 with higher stability and longer cycle life. The excellent performance was mainly attributed to the following factors: (1) formation of more cobalt active sites and uniform distribution; (2) inserting cobalt ions during the polymerization pyrolysis process, which facilitates the formation of catalytic active sites. Long and colleagues378 prepared GO-doped porous g-C3N4-loaded FeNi-MOF arrays as templates for the synthesis of FeNi-MOF@NCG double-template materials. Then, the hollow FeNi@NCG-T (T = 700°C) architecture, with its directional arrangement, facilitates charge transferred and increases the number of active sites, which could be obtained by calcining the FeNi-MOF@NCG template at high temperature. The as-synthetized FeNi@NCG-700 applied in zinc-air batteries exhibited high peak power density of 210.79 mW cm−2, high specific capacity of 784.84 mAh g−1, and long-term charge/discharge stability. Tian and colleagues379 designed and prepared low-cost and efficient nitrogen-doped porous carbon catalysts via an atomic doping strategy. The network structure coupled with fluorine and phosphorus atoms was prepared by pyrolyzing the mixture of poly3-fluoro aniline coating g-C3N4 with phytic acid. The prepared F/P-N-C-950 catalyst had a 3D porous layered structure, high specific surface area (505 m2 g−1), and abundant highly active sites under the synergistic effects of F, P, and N. The F/P-N-C-950 was applied in zinc-air batteries, and it exhibited high peak power density (138 mW cm−2), excellent specific capacity (821 mA h g−1), and good rate stability. Chen and colleagues380 synthetized Co3O4 (graphitizing the zeolitic imidazolate framework (ZIF)-67) encapsulated within nitrogen defect-rich g-C3N4 (denoted Co3O4@ND-CN). The prepared Co3O4@ND-CN was applied in zinc-air batteries, and it exhibited superior round-trip efficiency of ≈60% with long-term durability (over 340 cycles), high peak power density (99.04 mW cm−2), and specific capacity (731.6 mAh g−1 at 10 mA cm−2). Yang and colleagues381 reported a defect-rich, molecular doped 3D g-C3N4 FeNi NPs (FeNi/N-CNT), which FeNi NPs were prepared through thermal condensation polymerization of malazite and melamine, and coordination and pyrolysis of precursors containing Fe and Ni. The synthesized FeNi/N-CNT assembled zinc-air battery had a high peak power density (127 mW cm−2 at 203 mA cm−2), a high specific capacity (up to 838 mAh g−1 at 5.0 mA cm−2), and excellent stability in a charging/discharging cycle test (over 800 h at 5 mA cm−2). Meng and colleagues382 prepared different concave hollow carbon octadecahedron (CNQD/CoNBs), and g-C3N4 was added in the synthesis process of Co-based ZIF to adjust the stress balance of the framework structure in the pyrolysis process. This method greatly increased the specific surface area and the number of micropores of the material, exposed more active sites, and facilitated the reaction. The prepared CNQD/CoNBs exhibited high electrical power density (210 mW cm−2) and good long-term charge–discharge stability in zinc-air batteries. Wang and colleagues383 reported that Fe3N-N-C was prepared via a self-sacrificial template technique by using 2D g-C3N4. It presented a high specific surface area (529.486 m2 g−1), which provided more opportunities to construct more active sites and better N configuration. It showed high peak power density (144.83 mW cm−2 at 251 mA cm−2) and high discharging performance (794.04 mAh g−1 at 5 mA cm−2). Wang and colleagues384 reported that Fe3C/N,S-CNS was prepared by confining Fe3C NPs into N, S co-doped PCSs (Fe3C/N,S-CNS) via high-temperature pyrolysis. The 5-sulfosalicylic acid demonstrated as an ideal complexing agent for iron(ΙΙΙ) acetylacetonate, and g-C3N4 behaved as a nitrogen source. The prepared Fe3C/N,S-CNS was applied in a zinc-air battery, which exhibited high power density (163 mW cm−2) and ultralong stability by successively operating for 750 h.
3 CONCLUSION AND PROSPECTS
g-C3N4, a carbon material emerging after CNTs and graphene, holds great promise in the field of energy storage devices. It boasts abundant sources, a straightforward preparation process, and exceptional thermal and chemical stability, and exhibits potential for further research. Notably, g-C3N4 possesses notable advantages, such as a wealth of amino and Lewis basic groups, along with a high nitrogen density. Its composition and structure can be adjusted, rendering it widely applicable in energy storage devices to enhance their performance.
To address certain limitations of g-C3N4, such as poor conductivity and its small specific surface area, significant efforts have been made. Optimized modification techniques encompass morphology control, defect modification, mesoporous regulation, composite preparation, and conjugated system construction. Morphological and mesoporous regulation techniques are intended to improve the specific surface area of g-C3N4, augment active sites, and facilitate proton transport, although they do not fully resolve the conductivity issue. On the other hand, composite material preparation and conjugated system construction offer effective solutions to overcome poor conductivity.
The application and underlying mechanism of g-C3N4 in diverse energy storage devices necessitate the design and preparation of g-C3N4 materials with varying structures. By tailoring the structure of g-C3N4, its performance can be optimized to suit the specific requirements of each energy storage device.
Currently, the application of g-C3N4 in energy storage devices faces certain challenges. The electrochemical conversion and energy storage applications of g-C3N4 exhibit distinct reaction mechanisms, necessitating material modification and design tailored to enhance electrochemical performance. g-C3N4 has triazine or heptazine structure and a conjugated system. High energy storage device performance is achieved by constructing different dimensional structures, conjugated systems, and electron donor/electron acceptor systems, doping with different elements, and so forth. The available preparation and modification methods for g-C3N4 remain limited, however, prompting the need for the development of diverse techniques. For example, atomic layer deposition, electron beam irradiation, and so forth are used to prepare or modify materials to improve device performance.
Looking ahead, it is crucial to prioritize specific research directions to overcome these challenges. Besides the typical material-level structure and surface modifications, the integration of theoretical calculations and advanced characterization techniques can significantly contribute to materials research. Exploring the content and type of nitrogen in g-C3N4 materials holds promise for guiding material design and structural regulation. Additionally, studying the synergistic mechanisms of g-C3N4 composite materials is of great importance. For future laboratory-based investigations and commercialization of g-C3N4 composite materials in the energy storage field, some detailed prospects have been outlined as follows.
3.1 Electrochemical performance enhancement
- (a)
Surface modification: Developing surface modification techniques can enhance the interaction between g-C3N4 and electrolytes, facilitating ion diffusion and improving overall battery performance.
- (b)
Defect engineering: Tailoring the defects in g-C3N4 can enhance its charge transfer kinetics, boost specific capacity, and promote cycling stability in rechargeable batteries.
- (c)
Heteroatom doping: Introducing heteroatoms, such as boron, phosphorus, or sulfur, into the g-C3N4 structure can enhance its electronic conductivity, improve its ion storage capacity, and stabilize electrode/electrolyte interfaces.
3.2 Structural optimization
- (a)
Morphology control: Tailoring the morphology of g-C3N4, such as through nanosheet or NP engineering, can increase its surface area, shorten ion diffusion pathways, and improve charge/discharge rates.
- (b)
Pore engineering: Designing porous structures within g-C3N4 can enhance its specific surface area, promote electrolyte penetration, and facilitate ion transport, resulting in improved battery performance.
3.3 Composite formation
- (a)
Hybrid architectures: Combining g-C3N4 with other electrode materials, such as metal oxides, carbonaceous materials, or conductive polymers, can create composite electrode architectures with synergistic effects, enhancing energy storage capabilities, rate performance, and cycling stability.
- (b).
Binder optimization: Exploring suitable binders for g-C3N4-based electrodes can enhance adhesion, structural stability, and ion transport properties, contributing to improved battery performance.
3.4 Advanced characterization and modeling
- (a)
In situ/operando techniques: Employing advanced characterization techniques, such as in situ/operando spectroscopy and microscopy, can provide real-time insights into the structural and electrochemical dynamics of g-C3N4-based electrodes, aiding in the design and optimization of battery systems.
- (b)
Theoretical modeling: Utilizing theoretical modeling approaches, such as DFT, can provide valuable insights into the fundamental mechanisms governing the performance of g-C3N4-based rechargeable batteries, facilitating the design of novel electrode materials and interfaces.
3.5 Scalability and cost reduction
(a) Scalable synthesis: Developing scalable synthesis methods for g-C3N4 is crucial to enabling large-scale production, making it more accessible and economically viable for commercial battery applications. (b) Resource efficiency: Exploring sustainable and cost-effective sources for g-C3N4 synthesis, as well as developing efficient recycling and recovery processes, can contribute to reducing material costs and minimizing environmental impact.
These outlooks and prospects present avenues for further research and development to enhance the performance of g-C3N4 in rechargeable batteries. Continued exploration and optimization of these strategies, along with advancements in battery technologies, can pave the way for the practical application of g-C3N4 in next-generation energy storage devices.
ACKNOWLEDGMENTS
This work was supported by the Xianning City Program of Science & Technology (Grant No. 2022ZRKX051), the Hubei University of Science and Technology Doctoral Research Initiation Project (Grant No. BK202217), and the Science Development Foundation of Hubei University of Science & Technology (Grant Nos. 2021KF05 and 2021ZX14). Open access publishing facilitated by University of Wollongong, as part of the Wiley - University of Wollongong agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Xiaojie Yang completed his PhD at the School of Materials Science and Engineering at the Hubei University in 2021. Xiaojie Yang joined the Hubei University of Science and Technology in June 2021. His main research interest is the application of two-dimensional materials in energy storage and conversion.

Jian Peng is an associate research fellow at the Institute for Superconducting & Electronic Materials, University of Wollongong, where he received his PhD degree in 2022. Jian's main research field is on rechargeable battery systems, especially on sodium-ion batteries. Furthermore, he is the recipient of the 2021 Chinese Government Award for Outstanding Self-financed Students Abroad, Extraordinary Potential Prize, and Postgraduate Research Award 2021/2022 of the Australian Institute of Nuclear Science and Engineering. He was granted the ICDD 2021 Ludo Frevel Crystallography Scholarship Award.

Peng Yu completed his PhD in Resource and Environmental Chemistry at the China University of Geosciences under the guidance of Professor Fan Xia in 2021. During his doctoral studies, he also pursued research under the mentorship of Professor Jun He at the National Center for Nanoscience and Technology from 2018 to 2021. Following the completion of his Ph.D., he embarked on a postdoctoral fellowship in Professor Jiantao Han's research group at Huazhong University of Science and Technology. His research endeavors are primarily centered around the field of electrochemical energy storage and conversion.

Jiazhao Wang is a professor at the Institute for Superconducting and Electronic Materials, University of Wollongong, Australia. Her research activities focus on electrochemical energy storage in batteries, including Li/Na-ion batteries, Li/Na-air batteries, and Li/Na-sulfur batteries. She has won more than 30 research grants including 22 Australian Research Council (ARC) grants as a chief investigator (CI). She is the solo CI, first CI and APD fellow for five ARC Discovery Projects (DP) and five ARC Linkage Projects (LP).

Shixue Dou is a distinguished professor at the University of Wollongong, the founding director of ISEM. He received his PhD degree in Chemistry at Dalhousie University, Canada in 1984 and DSc at the University of New South Wales in 1998. He was elected as a Fellow of the Australian Academy of Technological Science and Engineering in 1994, and awarded the Australian Government's Centenary Medal in 2003 and Australian Order of Member in 2019. He is named as a highly cited researcher in materials science by Thomson Reuters. He is also a program leader in the Auto CRC 2020.




