Kinetics and Heat Exchanger Design for Catalytic Ortho-Para Hydrogen Conversion during Liquefaction
1 Erratum
P. J. Donaubauer, U. Cardella, L. Decker, H. Klein, Kinetics and Heat Exchanger Design for Catalytic Ortho-Para Hydrogen Conversion during Liquefaction, Chem. Eng. Technol. 2019, 42 (3), 669–679. DOI: https://doi.org/10.1002/ceat.201800345
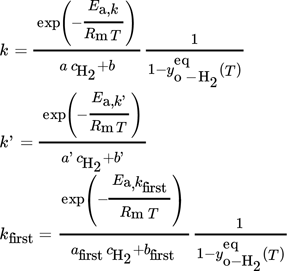 (6)
(6)2. The unit of b in Tab. 2 is incorrect, it should be b [×103 s]. The corrected table reads as follows:
|
Coefficient |
Group A |
Group B |
Group C |
Averagea |
|---|---|---|---|---|
|
Langmuir-Hinshelwood kinetics |
||||
|
Ea,k [J mol−1] |
n.a. |
–553.78±6.42 |
–618.78±5.21 |
–586.28 |
|
a [× 103 m3s mol−1] |
1.25±0.02 |
10.68±0.13 |
15.87±0.19 |
9.87 |
|
b [× 103 s] |
13.37±65.58 |
–109.74±96.79 |
–356.54±166.84 |
–144.50 |
|
Ea,k' [J mol−1] |
n.a. |
–26.77±6.43 |
68.75±2.28 |
20.99 |
|
a' [–] |
–2.08±0.03 |
–1.21±0.02 |
–0.22±0.00 |
–1.15 |
|
b' [mol m−3] |
358.52±106.87 |
170.16±11.80 |
–120.52±2.14 |
132.28 |
|
First-order kinetics |
||||
|
Ea,k [J mol−1] |
n.a. |
–362.73±5.69 |
–310.17±3.39 |
–336.45 |
|
afirst [× 103 m3s mol−1] |
0.88±0.00 |
3.00±0.03 |
2.15±0.02 |
2.20 |
|
bfirst [× 103 s] |
–99.38±12.54 |
8.65±24.56 |
58.55±16.25 |
–35.11 |
- a Ea-values for the Group A data are approximated via the mean values of Group B and C to calculate and adjust the average coefficients.




