Cost effectiveness of nonpharmacological prevention programs for diabetes: A systematic review of trial-based studies
Abstract
Trial-based economic value of prevention programs for diabetes is inexplicit. We aimed to review the cost-effectiveness of nonpharmacological interventions to prevent type-2 diabetes mellitus (T2DM) for high-risk people. Six electronic databases were searched up to March 2022. Studies assessing both the cost and health outcomes of nonpharmacological interventions for people at high-risk of T2DM were included. The quality of the study was assessed by the Consolidated Health Economic Evaluation Reporting Standards 2022 checklist. The primary outcome for synthesis was incremental cost-effectiveness ratios (ICER) for quality-adjusted life years (QALYs), and costs were standardized in 2022 US dollars. Narrative synthesis was performed, considering different types and delivery methods of interventions. Sixteen studies included five based on the US diabetes prevention program (DPP), six on non-DPP-based lifestyle interventions, four on health education, and one on screening plus lifestyle intervention. Compared with usual care, lifestyle interventions showed higher potential of cost-effectiveness than educational interventions. Among lifestyle interventions, DPP-based programs were less cost-effective (median of ICERs: $27,077/QALY) than non-DPP-based programs (median of ICERs: $1395/QALY) from healthcare perspectives, but with larger decreases in diabetes incidence. Besides, the cost-effectiveness of interventions was more possibly realized through the combination of different delivery methods. Different interventions to prevent T2DM in high-risk populations are both cost-effective and feasible in various settings. Nevertheless, economic evidence from low- and middle-income countries is still lacking, and interventions delivered by trained laypersons and combined with peer support sessions or mobile technologies could be potentially a cost-effective solution in such settings with limited resources.
Highlights
-
This review is the first to synthese trial-based economic evidence on nondrug prevention programs for type-2 diabetes mellitus (T2DM), and we identified that the US diabetes prevention program (DPP)-based and non-DPP-based programs are both cost-effective and feasible in preventing T2DM among high-risk populations.
-
The best cost-effectiveness outcomes were obtained through the combination of in-person and virtual delivery methods.
-
Interventions delivered by trained laypersons and combined with peer support sessions or mobile technologies could be a cost-effective solution especially in settings with limited resources.
1 INTRODUCTION
Type 2 diabetes (T2DM) is a serious chronic disease and has become epidemic worldwide, not only affecting 537 million adults but also placing 541 million adults at high risk at the same time. T2DM is related to high rates of disease-related morbidity and mortality, and it affects over three in four adults in low- and middle-income countries.1 According to estimates, T2DM accounts for over 966 billion US dollars in healthcare spending globally,1 and therefore cost-effective therapeutic and preventive measures are increasingly important to reduce this heavy burden from both economic and societal aspects. It was revealed that risk factors of T2DM include genetic, environmental, and behavioral factors such as family history, obesity, sedentary behavior, age, an unhealthy diet, and others. Of these risk factors, a certain proportion are modifiable and can be altered with healthier lifestyles. Therefore, international health organizations, including World Health Organization and National Health Service, advocate that the prevention of T2DM is better than cure.2, 3 Studies have also demonstrated the effectiveness of interventions targeted at preventing or delaying T2DM among high-risk populations in different countries.
Among different interventions to prevent diabetes, weight loss is regarded as an important component, since substantial research has revealed a high association between obesity and T2DM, and it could also compound other health problems and complicate the management of T2DM.4, 5 The most commonly used methods for high-risk people to control and lose weight are lifestyle changes and metformin. In comparison, lifestyle interventions have demonstrated better applicability in diverse populations and are superior to the use of metformin in older adults and lower body mass index (BMI) groups.6 Metformin may cause adverse reactions like diarrhea and nausea, and it may not be applicable in some patients owing to the risk of lactic acidosis.7 Besides, delivering lifestyle interventions may have long-term benefits for health and well-being, whereas glucose-lowering medications only suppress glucose while in use.8, 9 Hence, the cornerstone of existing programs to prevent diabetes remains to be lifestyle interventions based on modified diet and physical activity, to achieve weight loss in overweight subjects.6 Because prediabetes is asymptomatic and is frequently diagnosed incidentally, such as when people have blood tests for other reasons or participate in a proactive screening program, screening programs for high-risk people are proposed and emerging in the past few years. New mobile health (mHealth) and digital health technologies are also increasingly being applied and have shown effective in both the prevention and management of diabetes. Such applications include media campaigns supported by the community, secure messaging that reminds patients of medication adherence or lifestyle modification (LSM), web-based information, online health coaching, counseling, and so on.10-12
Previous studies have shown that lifestyle and metformin interventions not only delayed or prevented diabetes, but were also cost-effective or cost-saving no matter from a payer, health care system, or a societal perspective.6, 13, 14 Evidence also reveals that prediabetes screening was more cost-effective than no screening.15, 16 Such economic evidence could guide policymakers and insurers on how to design and implement different interventions, where different economic factors have to be considered in decision-making, including budget, cost and effectiveness of program, and equity of delivery. Despite existing clinical and economic evidence of interventions to prevent diabetes, the majority of these studies mainly focused on the comparison between lifestyle interventions and metformin, and a large proportion of them were modeling-based. There was also a lack of evidence evaluating the cost-effectiveness of digital health on T2DM prevention.10 In addition, the cost-effectiveness of different preventive interventions for T2DM could differ by types of hyperglycemia, settings, definitions of prediabetes, and intensities of interventions. Hence, there is a need for the study to review and synthesize the updated trial-based economic evidence for different interventions to prevent T2DM. Considering that patients’ habits are highly related to their progression of disease, and drug therapy should only be considered when lifestyle interventions fail or are not feasible, and also considering that there have been a previous review comparing lifestyle programs and metformin, this study would mainly focused on nondrug interventions.17-19
The primary objective of this review was to identify and synthesize cost-effective nonpharmacological interventions to prevent T2DM for high-risk people. The secondary objective was to assess the quality of reporting cost-effectiveness evidence and determine how different factors, such as intervention type, time horizon, and delivery method, would influence the results. We hope that this review would provide policymakers with advice on how to design and implement prevention strategies among high-risk populations.
2 METHODS
2.1 Search strategy
This study followed the guidelines of preferred reporting items for a systematic review and meta-analysis (PRISMA). The PRISMA checklist is shown in Supporting Information: Appendix 1. Literature published from inception to February 2022 was searched in PubMed, EMBASE, Science Direct, Web of Science, and NHS Economic Evaluation Database. Unpublished and gray literature were excluded, and the language of studies was restricted to English. Searching strategies were constructed with the following search terms included in Medical Subject Headings (MeSH), title, abstract, and keywords: “pre-diabetes” (relevant terms: diabetes, type 2 diabetes, T2DM, diabetes mellitus type 2, diabetes prevention), “economic evaluation” (relevant terms: cost, economic, cos-effectiveness, cost-effective, cost-utility, cost–benefit, cost-analysis) and “nonpharmacological interventions” (relevant terms: intervention, therapy, prevention, lifestyle, screening, digital health). Details about search terms and results are outlined in Supporting Information: Appendix 2. The initial search was done by Yongyi Xiong, and then the second reviewer Zhaohua Huo. All records identified from the searches were organized, and duplicate records have been excluded electronically and manually. Manual searches were extended to the references of relevant publications until no additional literature was found.
2.2 Study selection
This review included all trial-based economic evaluations of nonpharmacological interventions aimed at preventing diabetes for high-risk individuals who have prediabetes (a health problem with a blood glucose level that is higher than normal but not reach the threshold of diagnosed T2DM) or who have risk factors for developing T2DM, considering factors like age, BMI, and so on. Modeling-based studies were excluded because uncertainties of the hypothetical cohort could affect the quality of the evidence. Economic evaluations included cost-minimization analysis (CMA), cost-effectiveness analysis (CEA), cost-utility analysis (CUA), and cost–benefit analysis (CBA), which compared the costs and related outcomes between different interventions.20 Studies considered for inclusion refer to the population, intervention, comparison, outcomes and study criteria (Supporting Information: Appendix 3), and economic evaluations should have: (1) participants at high risk of developing T2DM, and a description of the method used to classify them as well as their baseline characteristics; (2) evaluated nondrug interventions aimed at high-risk individuals, such as lifestyle programs (with or without screening), digital health programs, and education; (3) interventions and comparison groups, such as standard care, placebo, no intervention, usual care and so on; (4) cost data; (5) quantitative outcomes, such as quality-adjusted life-year (QALY), disability-adjusted life-year (DALY), life-years gained or numbers needed to treat to prevent one case of diabetes.15 Unpublished studies, gray literature, review articles, articles focusing only on drug interventions, and articles focusing only on women with a history of gestational diabetes were excluded.
The two same reviewers initially screened for any duplicates, then for relevance in the titles and abstracts, and then for our inclusion and exclusion criteria in the full text. Any disagreement was first discussed between the two reviewers, and if it was still questionable, it would be referred to the third independent reviewer (Benjamin H. K. Yip).
2.3 Data extraction and cost adjustment
Using a standardized data extraction tool based on existing guidelines and other economic evaluation articles, relevant data were extracted by one investigator (Yongyi Xiong) and independently checked for accuracy by another investigator (Zhaohua Huo).20-22 We extracted the following data from included studies: study source, target population, intervention type, comparison, intervention media, discount rate, analytical time horizon, evaluation perspective, and reported cost-effectiveness outcomes. We used a web-based tool named the campbell and cochrane economics methods group and the evidence for policy and practice information and coordinating centre (CCEMG-EPPI-Centre) Cost Converter (version 1.6) to standardize costs into 2022 US dollars.23 The CCEMG-EPPI-Centre Cost Converter applies Gross Domestic Product (GDP) deflator index values and Purchasing Power Parities (PPP) conversion rates derived from the International Monetary Fund and the Organization for Economic Cooperation and Development, taking both inflation and per capita purchasing power into account.23
2.4 Quality assessment
After data extraction, the two same investigators independently assessed the quality of studies using the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) checklist, which can be used for any form of health economic evaluation.24 The quality of economic evaluation studies was assessed for each checklist item and graded as “Yes” or “No,” based on whether they reported information relative to the item or not. “N/A” indicates “not applicable to this paper” and “P” stands for “partially reported.” Considering we only considered trial-based studies in our analysis, we excluded the item 19 “distributional effects” from quality assessment, which is mainly applied in modeling-based studies, to increase comparability. The evaluation results were presented in two tables: one for checklist items and one for study compliance. We used green, red, yellow, and white colors to represent “Yes,” “No,” “P,” and “N/A,” referring to the previous study.16 Studies that met more than 80% of the criteria items would be deemed high quality, while those that met less than 60% would be considered poor.
2.5 Statistical analysis
Considering the anticipated disparity in the participants' study design, methods, interventions, and measures between studies, narrative synthesis was conducted.25 Descriptive analysis was conducted based on different studies, and evidence was synthesized based on different types of intervention, delivery methods, settings, and outcomes. The primary outcome of interest was the incremental cost-effectiveness ratios (ICERs) for quality-adjusted life years (QALYs), and the median and range of outcomes were reported. To improve comparability, we presented costs and cost-effectiveness results based on a standardized unit cost using a common international currency (US$) and base year (2022). According to the World Health Organization's guidelines,26 interventions were deemed cost-effective if the ICER was less than $50,000 every QALY saved, $50,000 per life-year-gain,27 or less than the relevant country's per capita GDP for the cost per DALY avoided.
3 RESULTS
3.1 Study selection
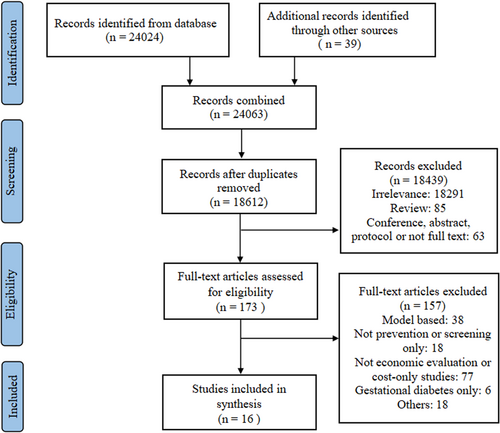
A total of 29,389 records were identified from six databases and we narrowed the focus to 234 full papers after screening the titles and abstracts. Eventually, 16 studies met our inclusion criteria and were included in the analysis. The process of study selection using the PRISMA flowchart is depicted in Figure 1.28
3.2 Study characteristics
The basic characteristics of the 16 included studies are summarized in Supporting Information: Appendix 4, based on the intervention feature and chronological order of publication. Despite large discrepancies in the study population, most studies recruited participants based on age, impaired fasting glucose, and BMI. All of the studies are trial-based, and one of them used economic modeling to extrapolate analyses to a lifetime horizon.29 The time horizon for the analysis ranged from 7 months to a lifetime. Three studies evaluated the cost-effectiveness of the US diabetes prevention program (DPP),13, 30, 31 a 3-year randomized clinical trial followed by a 7-year follow-up in the United States. One study evaluated the Support, Health Information, Nutrition, and Exercise (SHINE) trial, a telephone adaptation of the DPP lifestyle intervention with phone calls.32 One study evaluated the Fit Body and Soul (FBAS) trial, a faith-based lifestyle intervention based on the US DPP protocol.33 Six studies evaluated other types of lifestyle interventions, including diet and physical activity modification, counseling, behavioral coaching, and peer support.29, 34-38 One of them compared different intensities of the same intervention and concluded that intensive interventions were more cost-effective than the low-intensity program.29 These lifestyle interventions are mostly comprehensive and may both include educational sessions and health coaching. On the other hand, four studies evaluated interventions that only contained health education, and we classified them as a separate type. These educational interventions combined in-person and digital health technologies like phone calls, secure messaging, or online health education in service delivery, and two of them were merely virtually delivered by mobile phone messages or e-mails.39-42 The last study included evaluated the costs of a prediabetes screening program followed by lifestyle or metformin interventions.43 Studies were evaluated from different perspectives, including societal perspectives, healthcare perspectives, or payer perspectives. Most of the studies used a discount rate at 3% for costs and benefits. The cost-effectiveness outcomes of the included studies varied substantially, with the most reporting the ICER for QALY ($/QALY).
3.3 Quality assessment
The quality by items of the included studies is summarized in Figure 2, and the quality of each study is shown in Supporting Information: Appendix 4. The results are reported based on all the 28 items in the assessment tool.24 Item 19 was inapplicable for trial-based studies, and item 16 was evaluated in only two studies that used modeling to predict long-term benefits. As a result, none of the studies fulfilled all of the CHEERS 2022 checklist items (Supporting Information: Appendix 5). Among the 26 checklist items which are applicable for all included studies, 23 were evaluated with a rate of 80% or higher compliance in all studies, and the three weakest item checklist points were numbers 18, 25, and 21 (Figure 2). As for Item 18, only one study reported results for subgroups. Item 25 and Item 21 with lower compliance is about the effect/approach of engagement with patients and other stakeholders affected by the study.24 Except for these three updated items from the old version of CHEERS,44 the overall quality of included studies was high. In summary, 15 out of 16 of the included economic evaluations were deemed as high quality according to the checklist (with a compliance above 80% of the checklist items) and the remaining 1 was evaluated with moderate quality (Supporting Information: Appendix 5).

3.4 Cost-effectiveness of the interventions
Except for one group-based education intervention and one mobile phone messaging intervention, the majority of the interventions had effects comparable to the placebo or a “status quo” scenario, and were deemed as cost-saving or cost-effective under the defined thresholds in the original studies (Supporting Information: Appendix 4). Table 1 summarizes the main outcomes of different types of interventions. Nondrug interventions compared with the usual care had an ICER ranging from $54 to $121,302 per QALY gained. Among lifestyle interventions, DPP-based interventions are those following the US DPP core curriculum, including individual and group sessions, and were taught by trained healthcare professionals. Any adaptions based on the DPP protocol were also included in this group. It was shown that the US DPP-based interventions (with a median ICER of $27,077/QALY) had less cost-effective outcomes than non-DPP-based lifestyle interventions (with a median ICER of $1,395/QALY). It was also concluded that 100% of non-DPP-based lifestyle interventions were cost-effective or cost-saving, and only 75% of DPP-based lifestyle interventions were cost-effective. Regarding health education, ICERs were only reported in healthcare perspectives and ranged from $6258 to $121,302 per QALY. The ICERs for QALY of mobile phone interventions and the screening program were not reported. Regarding the second outcome of reduced diabetes incidence, DPP-based interventions inversely showed better effects in preventing T2DM (49.4%) when compared with non-DPP-based lifestyle interventions (2.1%).
| Type of interventionsa | Number of comparison pairs | Sample size | Median ICER (range), $/QALY from health care system perspective | Median ICER (range), $/QALY from societal perspective | Reduced diabetes incidence | Percentage of cost-effectivenessb |
|---|---|---|---|---|---|---|
| Nondrug intervention | 13 | 17,122 | $18,411 ($54–$121,302) | $26,838 ($168–$78,973) | 49.4%, 2.1% | 85%, cost-saving or cost-effective |
|
4 | 2765 | $27,077 ($17,445–$48,210) | $26,838 ($4383–$78,973) | 49.4% | 75%, cost-effective or cost-effective |
|
4 | 2476 | $1,395 ($54–$19,376) | $168, $40,012 | 2.1% | 75% cost-effective, 25% cost-effective |
|
4 | 11,881 | $6,258, $121,302 | NR | NR | 75% cost-effective |
| Mobile phone | 2 | 7906 | NR | NR | NR | 50% cost-effective |
| Screening | 1 | 1259 | NR | NR | NR | cost-effective |
- Note: For studies that did not conduct formal CEA; Percentage of cost-effectiveness outcomes based on “ICER: $/QALY” compared with the threshold, not other clinical outcomes; Costs are in 2022 US dollars.
- Abbreviations: NR, not reported; QALY, quality-adjusted life year.
- a DPP-based interventions are those that followed the US Diabetes Prevention Program (DPP) core curriculum, including individual and group sessions, and were taught by trained health care professionals. Any adaptions of DPP were also included in this group. Education refers to programs that exclusively use educational approaches and do not include coaching content. Mobile phone interventions are also a means of education, and we separated the interventions that are virtually delivered.
- b This table includes studies whose effect of the intervention was compared with the effect of placebo or a “status quo” scenario. The range of ICER is reported if there are three or more data points.
3.5 Associated factors of cost-effectiveness of interventions
We also stratified studies by different characteristics of intervention, including delivery mode (in-person, virtual, combined), time horizon (<3 years or ≥3 years), economic settings (high-income or low-income). Table 2 shows that interventions with a time horizon over 3 years had a higher ICER. The in-person and virtual combined delivery method appeared to have the best cost-effectiveness outcomes (with a median ICER of $6258/QALY) compared with in-person media (with a median ICER of $19,376/QALY). However, virtual media did not show up to have much evidence of cost-effective outcomes. Regarding economic settings, there is still a lack of studies conducted in low-and middle-income settings. It shows that interventions in low- and middle-income countries had lower ICER values than those in high-income countries.
| Feature of delivery | Number of comparison pairs | Sample size | Median ICER (range), $/QALY from health care system perspective | Median ICER (range), $/QALY from societal perspective |
|---|---|---|---|---|
| Mode of nondrug intervention | ||||
| In-person | 8 | 14,047 | $19,376 ($54–$48,210) | $26,838 ($168–$78,973) |
| Virtual | 2 | 7906 | NR | NR |
| In-person and virtual | 4 | 2210 | $6258 ($1,395–121,302) | $11,448 |
| Time horizon | ||||
| <3 years | 7 | 14,430 | $19,376 ($54–$121,302) | $168, $40,012 |
| ≥3 years | 6 | 5904 | $27,077 ($1,395–$48,210) | $26,838 ($4,383–$78,973) |
| Regional feature | ||||
| High-income | 9 | 7092 | $23,227 ($6,258–$121,302) | $33,425 ($4,383–$78,973) |
| Low-and middle-income | 4 | 13,242 | $1395, $54 | $168 |
- Note: This table includes studies whose effect of the intervention was compared with the effect of placebo or a “status quo” scenario. The range of ICER is reported if there are three or more data; Costs are in 2022 U.S. dollars.
- Abbreviations: NR, not reported; QALY, quality-adjusted life year.
Supporting Information: Appendix 6 shows the results of specific programs implemented in different income settings, also considering the attributes of interventions. In general, interventions conducted in low-and middle-income settings were more cost-effective compared with those in high-income settings. Meanwhile, interventions designed in low-and middle-income settings were more involved in lifestyle interventions by peer-support and group sessions, and most of the interventions were delivered in community settings by trained unprofessional workers or peer leaders. In high-income settings, most of the interventions were DPP-based and delivered by healthcare professionals. The low labor cost may contribute to the low ICERs of the intensive LSM interventions and peer-support lifestyle interventions in low-and middle-income settings, and they did not involve the cost of additional fitness equipment, which accounted for a big part of the nonmedical cost in the DPP. Finally, the threshold in each study varied, which could be determined by the local GDP, so we calculated ICERs as percent of GDP per capita in both the report year and in 2021 for comparison. It shows a trend of decreasing ICERs as percent of GDP in 2021 when compared with those in the report year, due to the economic growth over the years. When we set the willingness to pay (WTP) at $50,000, we found that more than 80% of the nondrug interventions were cost-effective or cost-saving.
4 DISCUSSION
4.1 Main findings
This review identified and synthesized the cost-effectiveness of different preventive interventions for high-risk populations of T2DM. We found that different types of interventions, including DPP-based and non-DPP-based lifestyle interventions, health education, as well as diabetes screening programs, were cost-effective when compared with usual care or no interventions. We also found that DPP-based lifestyle interventions were less cost-effective than non-DPP-based lifestyle interventions, and interventions with longer time horizons had a higher ICER, which contradicts previous literature.14, 45 One reason might be that our studies only included trial-based studies that focused mainly on short-term effectiveness, and there lacks long-term observation on these trial-based interventions. Besides trial-based analyses, there are also more than twice modeling-based studies that predict more than 10 years of effectiveness of DPP-based interventions. A previous systematic review included a large number of modeling-based studies, which have revealed the long-term cost-effectiveness of different programs to prevent diabetes. Another explanation could be related to the setting. Most of the DPP-based interventions were conducted in high-income settings while most of the non-DPP interventions were conducted in lower income settings. Cost of living, equipment, professionals, training, and specialist service is usually higher in high-income settings, leading to a higher ICER for outcome improvements.
Regarding delivery modes of intervention, we found that virtual education by mobile phone messaging did not show evidence of cost-effectiveness in the Bangladesh study, but demonstrated cost-effectiveness and acceptance in reducing risk factors for diabetes in young employees in the information technology industry in India.41, 42 This may be attributed to people's electronic product adherence and frequency of use in different settings, and further studies in other populations and settings should be considered. The noninvasive nature of the intervention, ease of administration, and low number of staff required for delivery are advantages of virtual interventions. Especially in Limaye's study in India, the weight loss recorded was comparable to that reported in other pragmatic lifestyle programs. Our synthesis also shows that the in-person and virtual united method appeared to be most cost-effective compared with in-person or virtual delivery only, so lifestyle advice through in-person and virtual combined approaches could be an efficient and potentially scalable intervention. Nevertheless, there was a lack of economic evidence on only mHealth or digital technologies interventions for T2DM prevention.6, 14
Finally, through our stratification of income level and supportive interventions, we found that lifestyle interventions delivered by trained unprofessional workers or laypersons and combined with peer-support sessions or mobile technologies could be potentially a cost-effective approach, especially for areas and populations faced with limited resources. There was still insufficient evidence to answer the question of how intensities of lifestyle interventions impact the results.
4.2 Limitations and implications
This review is the first to focus on and synthesize the trial-based economic evidence for nonpharmacological interventions to prevent T2DM, and also included most trial-based studies among similar topics. Overall, the quality of included studies was high when compared with modeling studies. However, there were a limited number of trial-based full economic evaluations or CEA studies. There were also insufficient studies in each subgroup, such as different types of prediabetes, interventions, settings, and so on, so we cannot compare the cost-effectiveness of different interventions in different subgroups. Also, a different definition of prediabetes and different settings limited the direct comparison between studies and external applicability of our findings. Economic evidence of different prevention programs in low-and middle-income countries was also insufficient. Further studies on the economic evidence of T2DM preventive intervention can focus more on various interventions in different populations and settings. Also, articles reporting information about the effect or approach of engagement with patients and others affected by the study, as well as the distributional effects of interventions, are important for decision-makers and further research.
Regarding the design and implementation of interventions, common technology-assisted interventions for T2DM or prediabetes including mobile phone messaging, conference calls, and telehealth are increasingly drawing more people's attention and have potential of leading to cost-effectiveness.46 Web-based applications or devices for weight loss or diet plans could also be considered as an intervention for diabetes prevention, and social networking or gamification elements to create a supportive or competitive online environment that may stimulate their autonomy for LSM, but unfortunately we have not found relevant economic evaluations.47 More economic evidence of this type of application is required.
5 CONCLUSION
Our findings support that various interventions for prediabetes or high-risk populations to prevent T2DM are cost-effective and practical in different settings. The estimated cost of lifestyle interventions varied widely between studies according to the intervention type, delivery method, intensity, application setting, and combination of other support interventions or not. The best cost-effectiveness outcomes were suggested through the combination of in-person and virtual delivery methods. Lifestyle interventions delivered by trained laypersons and combined with peer-support sessions or mobile technologies could be potentially a cost-effective approach, especially for areas and populations faced with limited resources. The cost-effectiveness of different applications of electronic technology in diabetes prevention requires further exploration, and continuous efforts are needed to produce full economic evidence alongside trials, especially in low-and middle-income countries.
AUTHOR CONTRIBUTIONS
Concept and design: Benjamin H. K. Yip, Yongyi Xiong, Zhaohua Huo. Acquisition of data: Yongyi Xiong, Zhaohua Huo. Analysis and interpretation of data: Yongyi Xiong, Zhaohua Huo. Drafting of the manuscript: Yongyi Xiong, Zhaohua Huo, Benjamin H. K. Yip. Critical revision of the paper for important intellectual content: Samuel Y. S. Wong, Yip. Statistical analysis: Yongyi Xiong, Zhaohua Huo. Administrative, technical, or logistic support: Benjamin H. K. Yip, Samuel Y.S. Wong.
ACKNOWLEDGMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This is a systematic review based on secondary data and was conducted in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
All data from this study are available in the publication.




