Altered asymmetries of resting-state MRI in the left thalamus of first-episode schizophrenia
Sha Liu and Zhenglong Guo contributed equally to this study.
Abstract
Background
Schizophrenia (SCZ) is a complex psychiatric disorder associated with widespread alterations in the subcortical brain structure. Hemispheric asymmetries are a fundamental organizational principle of the human brain and relate to human psychological and behavioral characteristics. We aimed to explore the state of thalamic lateralization of SCZ.
Methods
We used voxel-based morphometry (VBM) analysis, whole-brain analysis of low-frequency fluctuations (ALFF), fractional amplitude of low-frequency fluctuations (fALFF), and resting-state seed-based functional connectivity (FC) analysis to investigate brain structural and functional deficits in SCZ. Also, we applied Pearson's correlation analysis to validate the correlation between Positive and Negative Symptom Scale (PANSS) scores and them.
Results
Compared with healthy controls, SCZ showed increased gray matter volume (GMV) of the left thalamus (t = 2.214, p = 0.029), which positively correlated with general psychosis (r = 0.423, p = 0.010). SCZ also showed increased ALFF in the putamen, the caudate nucleus, the thalamus, fALFF in the nucleus accumbens (NAc), and the caudate nucleus, and decreased fALFF in the precuneus. The left thalamus showed significantly weaker resting-state FC with the amygdala and insula in SCZ. PANSS negative symptom scores were negatively correlated with the resting-state FC between the thalamus and the insula (r = −0.414, p = 0.025).
Conclusions
Collectively, these results suggest the possibility of aberrant laterality in the left thalamus and its FC with other related brain regions involved in the limbic system.
Highlights
- 1.
Experiments to explore the lateralization state of the left thalamus in schizophrenia.
- 2.
The thalamus is structurally and functionally impaired in schizophrenia.
- 3.
The limbic system may be linked to altered brain asymmetry.
1 INTRODUCTION
Schizophrenia (SCZ) is a severe, complex, and disabling mental disorder with multiple disorganized symptoms that affect how a patient thinks, acts, conveys emotions, perceives reality, and interacts with others.1 The disorder usually has a chronic disease course, with the first episode in early adulthood, and can eventually lead to mental disability.1 With a lifetime prevalence of about 1%,2 SCZ patients have a reduced life expectancy, accounting for a large health care burden worldwide.3 Although the neural mechanisms of the disease remain unclear, many studies have suggested widespread structural and functional alterations.4, 5
The thalamus is well known for its crucial role in the information processing of the brain.6 As an essential hub and key station of the cortico–cerebellar–thalamo–cortical circuit (CCTCC), the thalamus participates in various higher-order mechanisms of activity, including motivation, motor, emotion, and cognition processes.7 Consequently, damage to the thalamus might cause functional impairment in the CCTCC8 and potentially contribute to the manifestation of SCZ.9 Resting-state magnetic resonance imaging (rs-MRI) has been used in the study of psychiatric illnesses for decades as a viable technology for in vivo exploration of brain activity and connections. As such, many neuroimaging studies have provided robust evidence for a variety of thalamic anomalies in both first-episode and chronic SCZ, including reduced volume of the whole thalamus,10, 11 reduced gray matter volume (GMV) (mostly in the anterior, mediodorsal, and posterior regions),12 and even aberrant connectivity with the cortex, the basal ganglia, the cerebellum, and the hippocampus.13-15 Uncovering functional changes determined by abnormal GMV may help to understand the underlying pathogenic mechanism of SCZ.
Hemispheric asymmetries are a fundamental organizational principle of the human brain and relate to psychological and behavioral characteristics, and such atypical asymmetry patterns have been explored in many mental disorders including SCZ using structural and functional brain imaging.16, 17 For example, increased rightward asymmetry of the hippocampus has been found to be related to abnormal laterality of neural activity and connections in SCZ.18 Altered lateralization in other brain regions, including the left temporal,19 the pale nucleus, the caudate nucleus,20 and the basal ganglia,21 has also been reported. Specifically, the interruption of lateralization is linked to a fundamental symptom—the disconnection syndrome of SCZ.22, 23 Furthermore, this “syndrome” causes disruptions in functional connectivity (FC) in certain brain regions, and their laterality might underlie several symptom dimensions in SCZ.24 These findings imply that lateralization irregularities are linked to SCZ-specific psychophysiology. While previous studies have primarily focused on the lateralization of thalamic structures, few have focused on both the abnormal structural and functional lateralization of thalamic properties in SCZ.
Based on the findings from prior research, we hypothesized thalamus lateralization alternations in SCZ. In addition, we predicted that the asymmetry state of the thalamus would be related to the severity of symptoms in SCZ. The primary goal of this study was to profile the asymmetries of the thalamus in SCZ using structural and functional MRI (fMRI) techniques and unveil its correlation with psychiatric symptoms. Such efforts would deepen our understanding of the pathogenesis of SCZ, providing a basis for a neurobiological mechanism-based diagnosis of SCZ.
2 MATERIALS AND METHODS
2.1 Participants
Forty-four first-episode and drug-naïve right-handed inpatients with SCZ were recruited from the Department of Psychiatry, First Hospital of Shanxi Medical University, Shanxi, China. The inpatients were confirmed using the Structured Clinical Interview for the DSM-IV Axis I disorders-clinical version. The psychiatric symptoms of SCZ were assessed using the Positive and Negative Symptom Scale (PANSS), consisting of the positive, negative, and general psychopathology subscales. Matched 45 right-handed healthy controls (HCs) were also recruited from a volunteer panel to participate in the study. Moreover, HCs were screened for a history of medical or neuropsychiatric illness and a major neurological or psychiatric illness among their first-degree relatives. All the included participants had no safety contraindications or MRI, no previous or current neurological disease, no history of major medical or psychiatric comorbidities, no history of head injury with loss of consciousness, and no alcohol or drug abuse.
2.2 MRI acquisition
The functional and structural data were collected in the same session on a Siemens Trio 3.0 Tesla scanner. The participants were instructed to stay awake with their eyes closed, remain awake, not think of anything systematically, and keep their head motionless during scanning. After scanning, all the subjects reported that they had not fallen asleep or opened their eyes. Functional images were obtained using an echo-planar imaging sequence with the following parameters: 32 axial slices; repetition time (TR) = 2500 ms; echo time (TE) = 30 ms; matrix = 64 × 64; slice thickness = 3 mm with 1 mm gap; flip angle = 90°; field of view = 240 × 240 mm2; and voxel size = 3.75 × 3.75 × 4 mm.3 The resting-state scan lasted for 530 s and 212 volumes were acquired. T1-weighted anatomical images were acquired as a three-dimensional fast-spoiled gradient-echo sequence with the following parameters: TR = 2300 ms; TE = 2.95 ms; matrix = 240 × 256, 160 axial slices; slice thickness = 1.2 mm, no gap; flip angle = 9°; field of view = 225 × 240 mm2; and voxel size = 0.9375 × 0.9375 × 1.2 mm.3
2.3 The voxel-based morphometry (VBM) analysis
The VBM was performed the FMRIB Software Library voxel-based morphometry (FSL-VBM)25 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), which is an optimized VBM protocol26 implemented using tools from the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library27 (FSL 5.9.8, www.fmrib.ox.ac.uk/fsl). Although picture skull-striping, tissue class segmentation, and nonlinear translation to standard space stages were avoided, the suggested analytical methodology was followed. The normalized GM images produced with the fMRIPrep were averaged and flipped along the x-axis to create a left–right symmetric study-specific GM template. An equal number of images was captured with each setting to create an unbiased template (32 in total). At this stage, all original GM images were nonlinearly registered to the study-specific template and “modulated” to explain local constriction and/or extension caused by the nonlinear portion of the spatial transformation. The modulated GM images were then smoothed using an isotropic Gaussian kernel with a sigma = 3 mm (FWHM = 7 mm). Between-group differences in GM volume were determined using unpaired voxel-wise t-tests within the framework of the general linear model (GLM) accounting for age, gender, and white matter (WM) volume as a nuisance covariate.
2.4 fMRI: analysis of the amplitude of low-frequency fluctuations (ALFF) and the fractional amplitude of low-frequency fluctuations (fALFF)
Data processing and analysis for (resting-state) brain imaging software was used to analyze resting-state fMRI data.28 The first 10 volumes were eliminated. Slice timing was rectified and images were realigned spatially. Subjects with more than 2.0 mm maximum translation (in any direction of x, y, or z) or 2.0° maximum rotation during scanning were excluded (not present in this study). The Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) tool was then used to compute transformations from individual native space to the Montreal Neurological Institute (MNI) space, allowing for the separation of GM, WM, and cerebrospinal fluid (CSF). The preprocessed resting-state fMRI data were regressed as nuisance covariates with the WM signal, CSF, and Friston's-24 motion parameters.29 DARTEL was used to convert the collected fMRI data to MNI space (voxel size = 3 × 3 × 3 mm3), and then a Gaussian kernel of 5 mm full width at half maximum was used to decrease spatial noise.
The ALFF and fALFF were used in this study to characterize functional features in resting-state fMRI data because they have good sensitivity and specificity in detecting the degree of regional spontaneous brain activity.29 Each voxel's time series was translated into the frequency domain (ranging from 0 to 0.25 Hz), and the power spectrum was derived using the fast Fourier transform. The averaged square root of the power spectrum was acquired over frequencies ranging from 0.01 to 0.1 Hz, and this averaged square root was used to calculate the ALFF. The fALFF was calculated by dividing the power spectrum ratio of low-frequency fluctuations (0.01−0.1 Hz) by the power spectrum ratio of the full frequency range (0−0.25 Hz). Subsequently, all ALFF and fALFF were Z-score transformed to obtain zALFF and zfALFF to improve normality and eliminate signal differences.
2.5 Resting-state fMRI data FC pre-processing
Resting-state fMRI data were analyzed using the FMRI Expert Analysis Tool, version 5.98, which is a part of FSL. Motion correction with MCFLIRT,30 removal of non-brain structures with the Brain Extraction Tool, spatial smoothing with a Gaussian kernel with a 5 mm FWHM, and high-pass temporal filtering were used to pre-process resting-state fMRI data (cutoff: 100 s). The Improved Linear Model of the FMRIB was then used to accomplish time-series auto-correlation (FILM). Using a boundary-based registration procedure, resting-state fMRI data were coregistered to each participant's structural data using a boundary-based registration approach.31 They were then spatially standardized to the MNI-152 2 mm standard brain using an initial linear registration (FLIRT; FMRIB's linear image registration tool)30 and a nonlinear registration (FNIRT; FMRIB's nonlinear image registration tool).
2.6 Seed-based FC analysis
We selected the results of VBM as ROI for the FC analyses of resting-state fMRI data (the method of seed point selection is described in detail in Figure 3). We referred to the Harvard Oxford subcortical structural atlas (90% threshold) to define the seed in which we were interested. This atlas was registered in the MNI-152 standard space in order to maintain consistency across all subjects and align with typical fMRI analytic pipelines.32 To avoid the effects of CSF and WM signals, we also drew two masks representing CSF and WM. CSF and WM masks were drawn within a ventricular region of interest and a section centered in the WM in the MNI space. (CSF: inside a ventricular area in two hemispheres [9, −14, 20], [−9, −14, 20]; WM: region centered in the WM [33, −13, 27] and [−33, −13, 27]). These two masks were then registered back to each subject's individual space.
A GLM was performed for modeling the seed-whole-brain rs-FC by following the standard procedures.33 For each participant, we extracted the mean signals of the two seeds and the annoyance region for CSF and WM. In the first-level analysis, the mean signal within two seeds for each subject was modeled as an explanatory variable and the signals of CSF and WM were modeled as nuisance variables. Parameter estimates and variances were transmitted up to the group level using a mixed-effect FLAME technique (FLAME; FMRIB's local analysis of mixed effects). The differences in rs-FC between two groups were assessed using an independent-samples t-test. The statistical images were thresholded using cluster-forming correction determined by Z> 2.3 and a corrected cluster significance threshold of p < 0.05.
2.7 Statistical analyses
The SPSS for Windows statistical software was used to analyze the demographic and clinical data (version 23.0; IBM Corp., USA). The data features prompted the use of the Mann−Whitney U test and χ2 tests. The associations between neuroimaging measurements and clinical variables like the PANSS were investigated using Spearman's correlation analysis. The false discovery rate method with p < 0.05 was used for multiple-comparison correction.
3 RESULTS
3.1 Demographic characteristics and clinical symptoms
Table 1 shows the demographic and clinical features of the patients. There were no significant differences in age (Mann−Whitney U test, p = 0.325) and sex (χ2 test, p = 0.748) between SCZ and HCs.
| Variables | SCZ (n = 44) | HCs (n = 45) | p Value |
|---|---|---|---|
| Age (years), median (P25−P75) | 24 (17−32) | 24 (19−33) | 0.312a |
| Gender (F/M), n | 22/22 | 20/25 | 0.906b |
| PANSS | |||
| Total score, mean ± SD | 78.648 ± 13.630 | ||
| Positive score, mean ± SD | 19.864 ± 5.271 | ||
| Negative score, mean ± SD | 18.918 ± 6.759 | ||
| General, mean ± SD | 38.756 ± 8.043 |
- Abbreviations: F/M, female/male; HCs, healthy controls; SD, standard deviation; PANSS, Positive and Negative Syndrome Scale; SCZ, schizophrenia.
- a Mann−Whitney U-test.
- b χ2 test.
3.2 Cortical and subcortical GM volumes and their correlation with PANSS
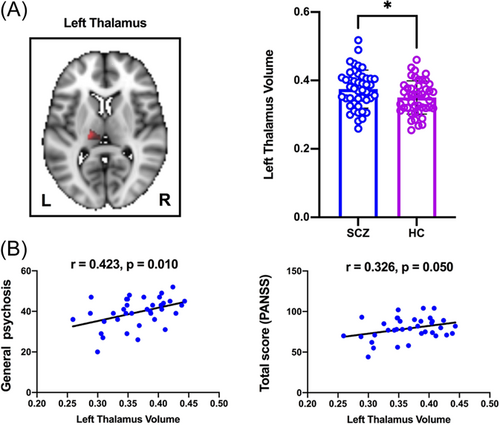
After controlling for age, gender, and total brain size, we discovered that GM volumes of the left thalamus were substantially larger in SCZ than in HCs (t = 2.214, p = 0.029) (Figure 1A). Correlation analyses showed that the left thalamus volume was positively correlated with general psychosis (r = 0.423, p = 0.010) and total PANSS scores (r = 0.326, p = 0.050) in SCZ (Figure 1B). Other subcortical regions, such as the nucleus accumbens (NAc), the left and right caudate nucleus, the left and right putamen, the left and right hippocampus, the left and right pallidum, and the right thalamus, showed no significant SCZ differences.
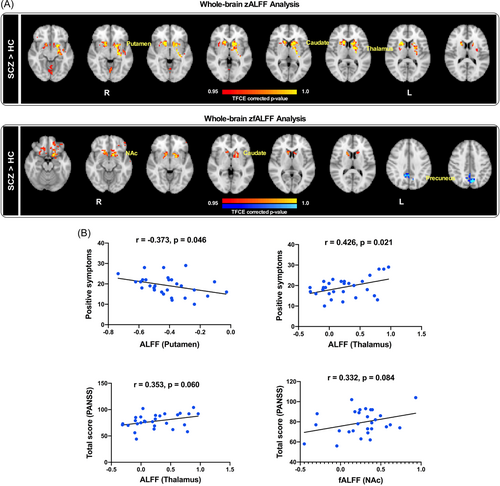
3.3 ALFF/fALFF differences between SCZ patients and HCs and its correlation with PANSS
In rs-fMRI, the SCZ group showed significantly higher zALFF in the putamen, caudate nucleus, and thalamus compared to the HC group. The SCZ group also showed significantly higher zfALFF in the NAc, caudate nucleus and lower zfALFF in the precuneus (Figure 2A). The positive symptoms were negatively correlated with the zALFF of the putamen (r = −0.373, p = 0.046) and positively correlated with the zALFF of the thalamus (r = 0.426, p = 0.021). Furthermore, the overall PANSS score was slightly positively linked to the zALFF of the thalamus (r = 0.353, p = 0.060) and the zfALFF of the NAc (r = 0.332 p = 0.084) (Figure 2B) (considering that if the sample size continues to expand, the correlation may be more significant). No additional significant associations were found between PANSS scores and the zALFF or zfALFF of other brain areas.
3.4 Seed-based FC differences between SCZ and HCs and their correlation with PANSS
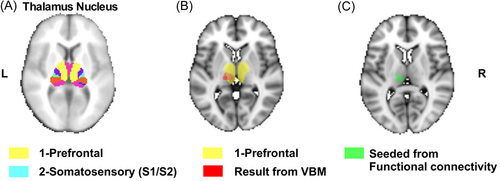
We then selected the seed location for seed-based FC analyses based on the following observations: (1) the selected seven thalamic nuclei provided in the FMRIB template for segmentation were chosen based on WM connections to cortical regions; (2) previous studies that have shown that the prefrontal cortex is central to the pathophysiology of SCZ;34 and (3) VBM results that showed that the GM volume between the two groups differed significantly in the thalamus nucleus, mainly connecting the prefrontal region (Figure 3). Accordingly, we chose the part in the FSL template to intersect with the abnormal structural region of the VBM as the seed.
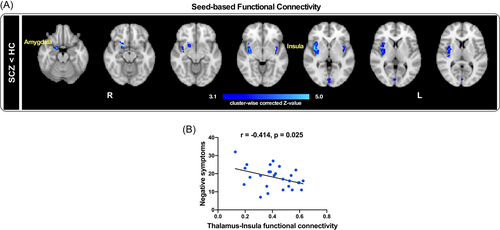
The thalamus showed significantly weaker resting-state FC with the amygdala and insula in SCZ than HCs (Figure 4A). Importantly, negative symptoms in PANSS scores were negatively correlated with the resting-state FC between the thalamus and the insula in SCZ (r = −0.414, p = 0.025, Figure 4B). No significant correlations were observed between thalamus-amygdala FC and the PANSS scores in SCZ. Furthermore, there were no significant correlations between thalamus-amygdala or thalamus-insula FC and the PANSS scores in HCs.
4 DISCUSSION
The purpose of this study was to investigate the link between the severity of symptoms of SCZ and structural and functional thalamic abnormalities. Consistent with our hypothesis, we obtained three main findings. First, although there were no differences in the whole-brain structure, we found substantial differences in the GMV of the left thalamus in the SCZ group versus the HC group, and our connectivity analysis found that the left thalamus volume was positively correlated with psychotic symptoms in SCZ. Second, the partial fMRI found ALFF differences in the putamen, caudate nucleus, and thalamus, and fALFF differences in the NAc, caudate nucleus, and precuneus between SCZ and HCs. Consistent with the structural MRI findings, the thalamus was also abnormal in the fMRI results, and connectivity analysis revealed that the ALFF of the thalamus was positively correlated with the positive symptoms in SCZ. Third, using FC analyses, we found that the thalamus showed significantly weaker resting-state FC with the amygdala and insula in SCZ; this was supported by follow-up analyses, which also found correlations with psychotic symptoms. Overall, these findings imply that altered thalamic structures and functions may be important in the pathogenesis and symptoms of SCZ.
4.1 The Left thalamic structure is abnormal in SCZ
The thalamus functions in brain systems to transmit information to the cerebral cortex and is critical in processing and coordinating sensory information.35 Previous conclusions on the changes of thalamic GMV in SCZ have been inconsistent. Most believe that the GMV of the thalamus is reduced, while other studies have found that the GMV of the thalamus does not change significantly or increase.36 Our study shows that thalamus volume in SCZ is larger than that in HCs, which is consistent with one previous study.37 Interestingly, previous meta-analyses have found that antipsychotic-naïve SCZ patients showed a greater thalamic volume change than medicated SCZ patients.38 Thus, drug effects and methodological discrepancies are likely to be responsible for thalamic volume aberrations. Moreover, rather than the voxel-based whole-brain analysis used in the current investigation, several earlier studies used volume measurements from a designated thalamic area.39 Further studies are needed to confirm the finding of increased thalamus volume. Additionally, findings of regional volume lateralization of the thalamus have been controversial and inconclusive.34, 40 Our study provides a possibility for altered lateralization of the left thalamus in SCZ, and these alterations are positively associated with psychiatric symptoms. These findings show that the pathophysiology of SCZ may be influenced by changes in the left thalamus.
4.2 Abnormal ALFF and fALFF in SCZ
SCZ patients have shown functional changes in spontaneous brain activity, and ALFF and fALFF of blood-oxygen-level-dependent signals produced from rs-fMRI can be used to quantify spontaneous brain activity with a high degree of reliability.41 Especially in terms of temporal stability, ALFF and fALFF are particularly prominent.42, 43 The striatum is an important part of the human brain's motor control and reward processes.44 As part of the striatum, the putamen is also involved in several areas of motor function, executive function, and emotion/motivation.45 While elevated ALFF in the putamen has been previously demonstrated in SCZ,46 our study further found that ALFF of the putamen is negatively correlated with positive symptoms. Moreover, the caudate nucleus on the other side of the striatum can plan and execute the behavior required for the target. Such goal-directed behavior can direct people's behavior to achieve the desired result.47 The thalamus plays a vital role in the bidirectional transmission of neuronal signals into or out of cortical areas, including the limbic system.48 Analogously, a survey of SCZ patients with persistent auditory verbal hallucinations showed increased ALFF in the bilateral thalamus.49 The NAc regulates information flow from the amygdaloid complex to the basal ganglia, the mediodorsal thalamus, and the prefrontal cortex as the reward network's central node. It also has a significant impact on cognitive, emotional, and psychomotor abilities in humans.50 As PANSS scores were positively correlated with the fALFF values of the NAc, our findings suggest that enhanced local functional activity in certain brain regions—including the striatum, the thalamus, the NAc, and the precuneus—can provide evidence for the extent of symptoms in subjects with SCZ.
4.3 Left thalamic FC abnormalities
Abnormal thalamic FC in SCZ has been the focus of ongoing research,51, 52 including investigations of the functional interactions between the thalamus and the cortex. Cheng et al. found that thalamic-sensory hyperconnectivity in SCZ was linked to negative symptom severity in a recent multisite investigation, while hyperconnectivity between the thalamus and the frontal cortex was linked to favorable symptom severity.53 In a meta-analysis, Li et al.54 discovered that thalamo-frontal hypoconnectivity and thalamo-sensory/motor hyperconnectivity were linked to the severity of positive symptoms in chronic SCZ, such as delusions, hallucinations, and suspiciousness. Overall, these studies show that thalamic FC disruption is linked to symptom severity, but few investigations have looked into unilateral thalamic FC changes. Our whole-brain FC analyses instead demonstrated that the left thalamus showed significantly weaker resting-state FC with the amygdala and the insula in SCZ, addressing the larger question of thalamic changes.
Patients with SCZ also show deficits in emotional perception and processing.55 Brain structures and networks involved in emotional processing are numerous and extensive in connectivity, including the prefrontal cortex, the orbitofrontal cortex, the ventral anterior cingulate gyrus, the amygdala, and the insula.56 The amygdala has been linked to negative emotions, especially fear and sadness, while hypoconnectivity between the amygdala and other brain regions implicates dysfunctional information processing related to negative emotions.57
Due to the insular cortex being located at the junction of the frontal, parietal, and temporal lobes, it plays a crucial role in cognitive, emotional, and somatosensory processes.58 The integration of the insula and the thalamus, which links information from different functional systems, confirms that altered thalamic-insula connectivity is unique to SCZ.59 In the present study, our correlation analysis revealed a strong negative association between left thalamus-insula FC and the PANSS negative symptom subscale scores, indicating that impairment in this specific FC may be linked to the development of negative symptoms of SCZ. In conclusion, the disruption of FC may provide preliminary evidence for the pathophysiological mechanism and diagnosis of SCZ.
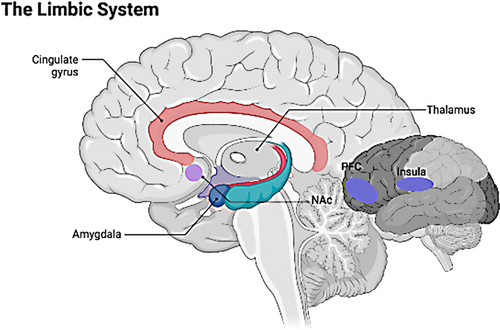
4.4 Limbic system
We performed a series of whole-brain fMRI analyses following abnormal left thalamic volume findings in SCZ subjects. Combining the above structural and fMRI results, we suspect that the pathogenesis of SCZ involves abnormalities in the limbic system. In addition to the cingulate cortex, the amygdala, and the hippocampus in the traditional concept, the limbic system also includes the NAc, the prefrontal cortex, the thalamus, the hypothalamus, and the insula.60, 61 For instance, the amygdala and the orbitofrontal cortex are mainly involved in emotional and reward-related processing; the hippocampus is a critical structure in episodic memory. However, the FCs of different limbic structures in emotion, memory, and action are worthy of investigation. Previous studies have found reduced resting-state FC between the limbic system (including the parahippocampus, the hippocampus, and the posterior cingulate cortex) and the thalamus.62 This lower resting-state FC affected a wide variety of thalamic subregions, which is consistent with a previous thalamic research in SCZ that revealed a wide range of thalamic functional dysconnectivity in the CCTCC pathway.6 We found similar results in whole-brain FC using the left thalamus as the seed point, further supporting our hypothesis of altered thalamic lateralization in SCZ. Thus, based on the abnormal asymmetry of the left thalamus, our study provides a new perspective that the disturbance of the limbic system may be an important signature for early pathophysiological mechanisms and psychiatric symptoms of patients with SCZ (Figure 5).
Our study was not without some limitations. First, the sample size was relatively small, and the age range of the included population is relatively concentrated. Accordingly, our findings need to be verified in a better-matched and larger sample. Second, the PANSS was the sole rating scale used to examine clinical symptoms, and future studies should incorporate other cognitive and symptom assessments. Third, as a study of brain lateralization, it is insufficient to only include right-handed patients, and future work should also include left-handed patients.
In summary, our study demonstrates altered left thalamic lateralization in right-handed SCZ, and that these changes may be related to the entire limbic system. These abnormalities may result from SCZ disease progression or a compensatory result of SCZ. Future studies with larger sample sizes and additional methods such as diffusion tensor imaging and task-based fMRI are required to further examine the role of the limbic system in the development and treatment of SCZ.
AUTHOR CONTRIBUTIONS
Yong Xu designed and supervised this study. Sha Liu organized the experimental data. Hong Li performed the statistical analysis. Zhenglong Guo and Sha Liu wrote the first draft of the manuscript. Hong Li and Yong Xu contributed to revision of the manuscript. Hongbao Cao and Ruize Liu provided valuable advice and revised the manuscript. Long Cheng, Xiaodong Hu, and Jianying Li assisted with recruitment of the participants. All authors discussed the results and commented on the manuscript.
ACKNOWLEDGMENT
The authors are grateful to all the participants of this study. This study was supported by the National Natural Science Foundation of China (81701326 and 81971601), the National Key Research and Development Program of China (2016YFC1307004), the Multidisciplinary Team for Cognitive Impairment of Shanxi Science and Technology Innovation Training Team (201705D131027), the Special Project of Scientific Research Plan Talents of Shanxi Provincial Health Commission (2020081), the Shanxi Province Overseas Students Science and Technology Activity Funding Project (20200038), and the Shanxi Provincial Science and technology achievements transformation and guidance project (201904D131020).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University (ChiCTR1900025838). Informed written consents were obtained from all the participants and their guardians.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




