Continuation Versus Interruption of Oral Anticoagulation During TAVI: A Systematic Review and Meta-Analysis Oral Anticoagulation Management in TAVI
ABSTRACT
Patients undergoing transcatheter aortic valve implantation (TAVI) often require long-term oral anticoagulation (OAC), but it is unclear whether to continue or interrupt OAC during the procedure. This meta-analysis compares clinical outcomes of continuing versus interrupting OAC during TAVI. PubMed, Embase, and Cochrane Central databases were searched from inception to September 2024 for studies comparing continuation versus interruption of OAC in patients undergoing TAVI with an indication for OAC, including vitamin K antagonists and direct oral anticoagulants. Risk ratios (RR) with 95% confidence intervals (CI) were pooled using a random-effects model. Sensitivity analysis was performed using the Hartung-Knapp-Sidik-Jonkman method. Three studies were included, one randomized controlled trial and two cohort studies, with 2773 patients, of whom 1314 (47.4%) continued OAC during TAVI. At a 30-day follow-up after TAVI, there were no significant differences between groups in all-cause mortality (RR 0.74; 95% CI 0.45–1.20; p = 0.22), any bleeding (RR 1.08; 95% CI 0.81–1.43; p = 0.60), and major bleeding (RR 0.90; 95% CI 0.67–1.21; p = 0.48). However, the continued OAC group was associated with a lower stroke rate (RR 0.65; 95% CI 0.42–1.01; p = 0.053), also attested after a sensitivity analysis (RR 0.65; 95% CI 0.47–0.90; p < 0.03). In patients with an indication for OAC undergoing TAVI, uninterrupted anticoagulation is associated with similar thrombotic and hemorrhagic outcomes compared to interrupted OAC. Stroke risk was lower in the continued OAC group, with a significant reduction, as demonstrated in sensitivity analysis.
Abbreviations
-
- CI
-
- confidence interval
-
- DOAC
-
- direct oral anticoagulants
-
- GRADE
-
- Grading of Recommendations, Assessment, Development, and Evaluation
-
- HKSJ
-
- Hartung-Knapp-Sidik-Jonkman
-
- OAC
-
- oral anticoagulation
-
- PRISMA
-
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
-
- RCT
-
- randomized controlled trial
-
- ROBINS-I
-
- Cochrane tool for assessing risk of bias in non-randomized studies
-
- RoB 2
-
- Cochrane tool for assessing risk of bias in randomized trials
-
- RR
-
- risk ratio
-
- TAVI
-
- transcatheter aortic valve implantation
-
- VARC-2
-
- Valve Academic Research Consortium-2
-
- VARC-3
-
- Valve Academic Research Consortium-3
-
- VKA
-
- vitamin K antagonists
1 Introduction
Transcatheter aortic valve implantation (TAVI) has become a first-line therapeutic approach for patients with severe symptomatic aortic stenosis, particularly in individuals considered high-risk, such as the elderly and those with significant comorbidities or previously degenerated prostheses [1]. However, despite significant technical advancements over the years, thromboembolic and bleeding complications remain substantial clinical concerns during the periprocedural period [2].
Approximately one-third of patients undergoing TAVI for the treatment of severe aortic stenosis have an indication for long-term oral anticoagulation (OAC) with a vitamin K antagonist (VKA) or a direct oral anticoagulant (DOAC), predominantly due to atrial fibrillation [3]. Managing anticoagulant therapy in these patients poses a significant clinical challenge, as discontinuing anticoagulation can reduce the risk of bleeding but increase the likelihood of thromboembolic events. Conversely, continuing anticoagulation can lower thromboembolic risk but may come with a potentially higher risk of bleeding [2].
International guidelines, such as those from the American College of Chest Physicians and the American College of Cardiology/American Heart Association, often recommend interrupting anticoagulation in procedures with a high risk of bleeding, such as TAVI [4, 5]. However, the optimal anticoagulation strategy during TAVI remains unclear. Recent observational data suggest that continuing anticoagulation during TAVI may be safe, with some studies indicating a lower incidence of thromboembolic events without a significant increase in major bleeding risk [6, 7]. Nevertheless, these analyses are limited by relatively small sample sizes and wide confidence intervals (CI), creating uncertainty regarding the safety and efficacy of this approach.
Therefore, our objective was to conduct a systematic review and meta-analysis encompassing all available studies, comparing the safety and efficacy of continuing versus interrupting OAC during TAVI, evaluating key outcomes such as all-cause mortality, stroke, and minor and major bleeding complications within 30 days post-procedure.
2 Methods
2.1 Eligibility Criteria
Studies were included in this systematic review and meta-analysis if they met the following criteria: (1) RCTs or observational studies, (2) comparing continuation versus interruption of OAC (including VKA and DOAC) during TAVI, (3) involving patients with an indication for OAC undergoing TAVI, and (4) reporting outcomes of interest such as all-cause mortality, stroke, any bleeding, and major bleeding. Accordingly, studies were excluded if they (1) lacked a control group for comparison, (2) did not provide clear stratified data, (3) were case reports, letters, or comments, (4) were written in languages other than English, or (5) involved overlapping patient populations, identified by studies conducted over the same period with common authors or study centers. In such cases, only the study with the larger sample size was included. No restrictions were applied regarding the publication date or follow-up duration.
2.2 Search Strategy and Data Extraction
We performed a structured search of PubMed (MEDLINE), Embase, and Cochrane Central Register of Controlled Trials databases from inception until September 12th 2024, using the following search terms: (“transcatheter aortic valve replacement” OR “transcatheter aortic-valve replacement” OR TAVR OR “transcatheter aortic valve implantation” OR “transcatheter aortic-valve implantation” OR TAVI) AND (“oral anticoagulation” OR “vitamin K antagonist” OR “vitamin K antagonists” OR warfarin OR “direct oral anticoagulant” OR “direct oral anticoagulants” OR apixaban OR dabigatran OR edoxaban OR rivaroxaban). The search was adapted for each database as necessary. Additionally, reference lists of all included studies and relevant systematic reviews were manually searched for any additional eligible studies. Data extraction was conducted independently by two authors (A.L.C.V and J.M.B.M), each following the predefined criteria. Any disagreements between the authors were resolved through discussion, and reasons for study exclusion were documented. This meta-analysis protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under protocol CRD42024588855.
2.3 Outcomes
The primary outcome of interest was (1) all-cause mortality. Secondary outcomes included: (2) stroke; (3) any bleeding; and (4) major bleeding. These outcomes were defined according to the study authors' definitions, following the Valve Academic Research Consortium-2 (VARC-2) criteria and the Valve Academic Research Consortium-3 (VARC-3) guidelines, and they all occurred within 30 days post-TAVI [8, 9].
2.4 Quality Assessment
The risk of bias and quality assessment in randomized studies were evaluated with version 2 of the Cochrane Risk of Bias assessment tool (RoB 2) [10]. We collected data on outcomes based on intention-to-treat analysis. Non-randomized studies were assessed with the Risk of Bias in Non-randomized Studies of Interventions tool (ROBINS-I) [11]. Two independent authors (C.K.F and P.L.F.V) completed the risk of bias assessment. Disagreements were resolved through a consensus after discussing the reasons for the discrepancy (J.M.B.M.).
Two independent authors (P.A.M.G.S and R.S.B) assessed the level of certainty of the evidence in this meta-analysis using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) handbook guidelines, with categorizations ranging from very low to high [12]. Any disagreements were resolved through discussion with a third author (J.M.B.M).
2.5 Statistical Analysis
This systematic review and meta-analysis were conducted in accordance with the methodological guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions and in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13, 14]. All analyzed outcomes were dichotomous, and risk ratios (RR) with 95% CI were used to compare treatment effects. Heterogeneity was assessed using the I² statistic and Cochran's Q test. Outcomes were considered to have low heterogeneity if p > 0.10 and I² < 25%, moderate heterogeneity if I² was between 25% and 75%, and high heterogeneity if I² > 75%. Statistical significance was defined as p < 0.05.
We used the DerSimonian and Laird random-effects model to account for the expected variability between studies, particularly given the inclusion of observational studies [15]. However, to ensure the robustness of our findings and address any uncertainty in the effect estimates, we performed an additional sensitivity analysis using the Hartung-Knapp-Sidik-Jonkman (HKSJ) method, as recommended in the Cochrane Handbook (Section 10.10.4.4) [13]. This method is recommended for meta-analyses with a small number of studies or in cases where there is low apparent heterogeneity but potential uncertainty due to study sizes. The HKSJ analysis was conducted to confirm the robustness of our results by adjusting for potential imprecision. All statistical analyses were performed using R statistical software version 4.2.2 (The R Foundation for Statistical Computing), utilizing the “meta” and “metafor” packages, along with RStudio version 2024.04.2 + 764 (RStudio Team) [16-18].
3 Results
3.1 Study Selection and Baseline Characteristics
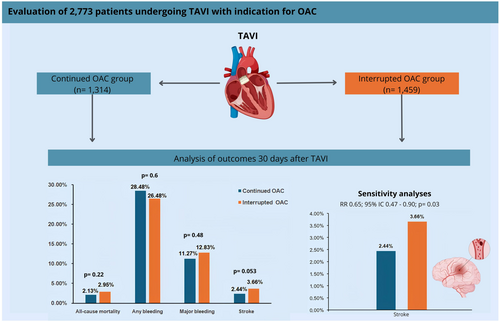
As shown in Figure 1, the search strategy identified 1949 studies. After removing duplicate records and excluding studies based on the title/abstract triage, eight studies remained for full-text assessment. Ultimately, three studies were included in our analysis: one randomized controlled trial and two observational studies, comprising a total of 2773 patients. Of these, 1314 (47.4%) continued OAC during TAVI, while 1459 (52.6%) interrupted OAC. The mean age of the patients ranged from 80 to 82 years, and 46.9% (n = 1301) were female. The follow-up period for all included studies was 30 days. The main characteristics of the study populations are presented in Table 1. The studies excluded from the meta-analysis and the reasons for their exclusion are detailed in Supporting Information S1: Table 2.
| Number of patients n | Female n (%) | Age (years) | CHA2DS2-Vasc scorea | Atrial fibrillation n (%) | Hypertension n (%) | Diabetes Mellitus n (%) | Previous myocardial infarction n (%) | Previous ischemic stroke n (%) | OAC type n | OAC type n | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Journal | Design | Country | Follow-up (days) | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER | CONT | INTER |
| Mangner et al. [17] | 2019 | AJC | Prospective cohort | Germany | 30 | 299 | 299 | 164 (54.8) | 172 (57.5) | 80.5 ± 5.2 | 79.6 ± 5.2 | 5.4 ± 0.7 | 5.6 ± 0.7 | NA | NA | 291 (97.3) | 289 (96.7) | 126 (42.1) | 162 (54.2) | 27 (9) | 46 (15.4) | 48 (16) | 39 (13) | DOAC (182)/VKA (117) | DOAC (0)/VKA (299) |
| Brinkert et al. [18] | 2021 | JACC | Retrospective cohort | European Multicenter | 30 | 584 | 733 | 287 (49) | 382 (52) | 81.6 ± 5.2 | 82 ± 5.9 | 5 ± 1.5 | 5 ± 1.5 | 500 (97) | 644 (94) | 525 (90) | 664 (91) | 204 (35) | 277 (38) | NA | NA | 90 (15) | 118 (16) | DOAC (290)/VKA (294) | DOAC (222)/VKA (511) |
| POPular PAUSE TAVI [4] | 2024 | NEJM | RCT (mul-center, open-label, non-inferiority clinical trial) | International Multicenter | 30 | 431 | 427 | 158 (36.7) | 138 (32.3) | 81.4 ± 5.6 | 80.9 ± 6.2 | 4.5 ± 1.4 | 4.4 ± 1.4 | 414 (96.1) | 406 (95.1) | 339 (78.7) | 322 (75.4) | 128 (29.6) | 123 (28.8) | 61 (14.2) | 75 (17.6) | 39 (9.0) | 51 (11.9) | DOAC (355)/VKA (76) | DOAC (349)/VKA (78) |
- Note: Values are n (%), mean ± SD, or median (interquartile range).
- Abbreviations: AJC, The American Journal of Cardiology; CONT, continued; DOAC, direct oral anticoagulants; INTER, interrupted; JACC, Journal of the American College of Cardiology; NA, not applicable; NEJM, The New England Journal of Medicine; RCT, randomized controlled trial; VKA, vitamin K antagonists.
- a CHA2DS2-Vasc score is an index of the risk of stroke in patients with atrial fibrillation.
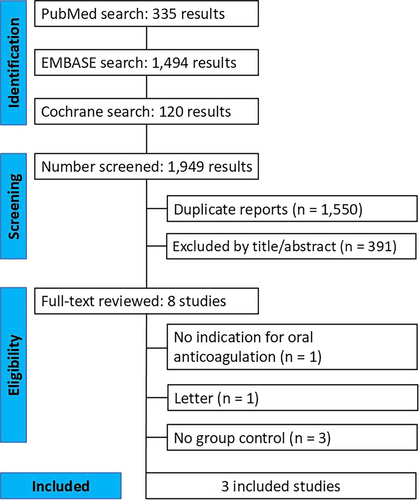
3.2 Primary Outcome
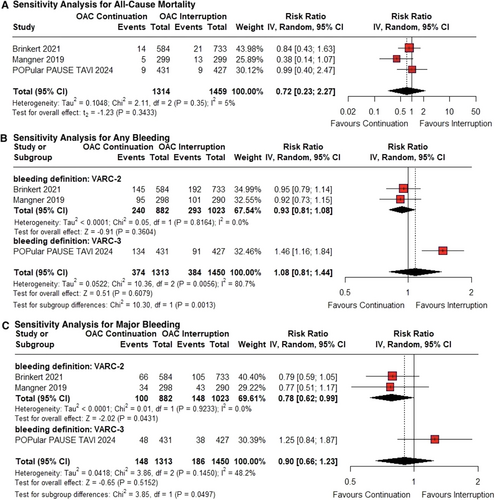
At 30 days post-TAVI, all-cause mortality occurred in 2.13% (28/1314) of patients in the continued OAC group, compared with 2.95% (43/1459) in the interrupted OAC group. There was no significant difference in all-cause mortality between the groups (RR 0.74; 95% CI 0.45–1.20; p = 0.22; I² = 5%; Figure 2).
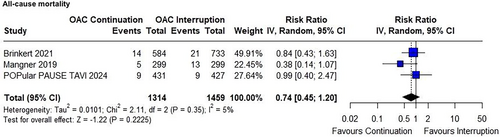
3.3 Secondary Outcomes
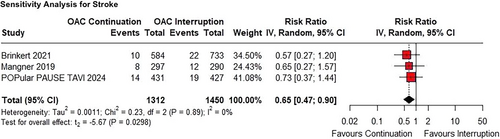
The risk of stroke in the continued OAC group compared to the interrupted OAC group did not reach significance in the primary analysis (RR 0.65; 95% CI 0.42–1.01; p = 0.053; I² = 0%; Figure 3).
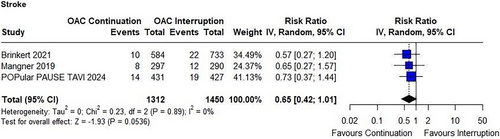
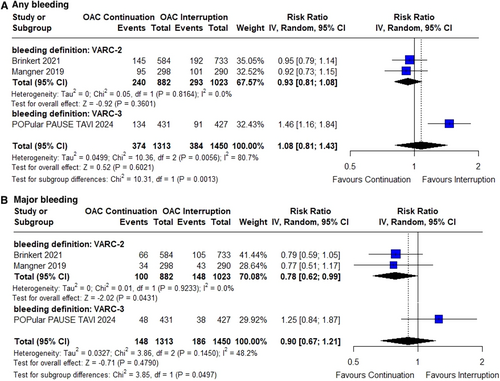
There were no significant differences between the groups in the incidence of any bleeding (RR 1.08; 95% CI 0.81–1.43; p = 0.60; I² = 81%; Figure 4A) or major bleeding (RR 0.90; 95% CI 0.67–1.21; p = 0.48; I² = 48%; Figure 4B). However, subgroup differences in major bleeding became evident when results were pooled by bleeding definition (VARC-2 vs. VARC-3).
3.4 Sensitivity Analyses
Concerning the risk of stroke, the sensitivity analysis showed a significant reduction, indicating that the continued OAC group had a lower incidence of stroke compared with the interrupted group (RR 0.65; 95% CI 0.47−0.90; p < 0.03; Figure 5). In terms of all-cause mortality, the results remained non-significant (RR 0.72; 95% CI 0.23−2.27; p = 0.34; I² = 5%; Figure 6a). Similarly, there was no significant difference in the occurrence of any bleeding (RR 1.08; 95% CI 0.81−1.44; p = 0.61; I² = 81%; Figure 6b), or major bleeding (RR 0.90; 95% CI 0.66−1.23; p = 0.52; I² = 48%; Figure 6c). However, subgroup differences in major bleeding were observed depending on the bleeding definition used (VARC-2 vs. VARC-3).
3.5 Risk of Bias Assessments
The ROBINS-I tool was applied to evaluate Mangner et al. and Brinkert et al., both of which showed an overall moderate risk of bias [6, 7]. Mangner et al. [6] had moderate risk due to confounding factors and deviations from the intended interventions, while Brinkert et al. [7] demonstrated moderate bias only in deviations from the intended interventions. Both studies exhibited a low risk of bias across all other domains, reflecting a generally sound methodology, despite limitations related to confounding and intervention deviations (Figure 7A).
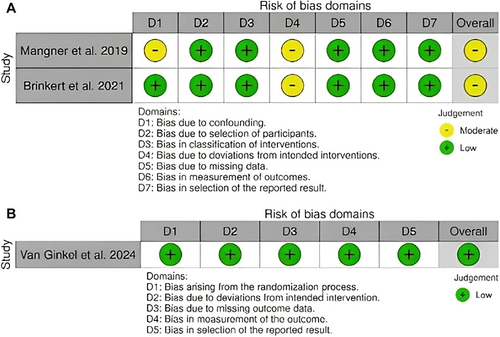
The ROB-2 assessment of Van Ginkel et al. [2] shows a low risk of bias across all domains, including bias arising from the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Overall, the study was determined to have a low risk of bias [2] (Figure 7B).
According to the GRADE evaluation, the risk of bias was considered serious for all outcomes due to the inclusion of observational data in the overall analysis, as well as imprecision related to wide CI and a limited number of events. The certainty of evidence for hemorrhagic outcomes was very low, with moderate and high heterogeneity impacting confidence in these results (I² = 48% for major bleeding and I² = 81% for any bleeding, respectively). For stroke prevention in the continued OAC group, the evidence was classified as low certainty, downgraded due to the limited number of studies and the lack of statistical significance in the primary analysis (Supporting Information S1: Table 1).
4 Discussion
In this systematic review and meta-analysis, we compared the continuation and interruption of OAC (including VKA and DOACs) in patients undergoing TAVI. The main findings were: no significant difference was found between the groups in terms of (1) all-cause mortality at 30 days; (2) rate of stroke; (3) rate of any bleeding; (4) rate of major bleeding. However, in the sensitivity analysis, the rate of stroke was significantly lower in the continued OAC group compared to the interrupted group (5) (Central illustration 1).
The incidence of any bleeding was below 2%, with major bleeding around 1.2%, primarily related to the vascular access site, as typically expected in this procedure. This rate is lower than those reported in previous studies and aligns with the trend of exponentially decreasing bleeding complications observed over the past two decades [19]. This improved safety concerning bleeding risk can be attributed to the increased expertise of interventionists in managing challenging access-site situations, as well as the widespread use of mechanical hemostasis devices [20]. These preventive measures likely explain why, even with continued anticoagulation, there was no increased risk of any or major bleeding.
Our findings align with similar studies on percutaneous cardiac procedures, such as pacemaker and implantable cardioverter-defibrillator placements, which also carry a risk of puncture-site complications and are often performed on older, high-bleeding-risk populations [21]. Randomized studies and meta-analyses have shown that in these scenarios, continued anticoagulation does not increase the risk of bleeding or mortality. Conversely, in patients where anticoagulation was interrupted and bridging with heparin was necessary, particularly those at high risk for thromboembolic events, the incidence of pocket hematoma was higher [22, 23].
While previous evidence and our study suggest that continuing anticoagulation is safe, the randomized POPular PAUSE TAVI trial reported a different outcome2. Although it found no difference in major bleeding between continued and interrupted anticoagulation, it did observe an increased risk of any bleeding in patients who remained on therapy, primarily driven by minor bleeding (VARC-3 type 1, similar to BARC 2). The increased bleeding risk in patients on continued anticoagulation may have been influenced by the type of anticoagulant used: 62.94% of patients in the continuation group used DOACs, while 60.86% in the interruption group used VKAs. Although minor bleeds carry less prognostic weight than major bleeding and may be clinically less impactful, their increased frequency in patients continuing OAC highlights the need to balance the potential reduction in thromboembolic events with a higher incidence of lower-severity bleeding. This trade-off underscores the importance of individualized risk assessment—considering factors such as access-site management and patient comorbidities—so that clinicians can optimize both safety and efficacy when deciding whether to continue or interrupt OAC in the TAVI population. Finally, although the included studies applied either the VARC-2 or the updated VARC-3 definitions, these frameworks are sufficiently aligned to ensure the validity of our analysis, as both distinguish minor from major bleeding in a clinically relevant manner.
Our study's stroke incidence of ~3% is consistent with prior reports and underscores stroke as a major complication of TAVI [24]. The difference in stroke risk between continued and interrupted OAC was not statistically significant, and the point estimate favored continued anticoagulation. We caution, however, against overinterpreting a nearly significant p value (p = 0.053) as a “trend” toward benefit, as Wood et al. 2014 emphasize that such interpretations can be misleading [25]. Importantly, our sensitivity analysis using the HKSJ method—chosen because our meta-analysis included only three studies, a setting in which HKSJ more reliably accounts for uncertainty in random-effects estimates—actually strengthened the finding of lower stroke risk in the continuation group [13]. This result is biologically plausible, given the high baseline thromboembolic risk in TAVI patients and the procedural endothelial injury that triggers coagulation cascades and platelet activation, heightening inflammation and thrombogenicity [26-28].
4.1 Limitations
Therefore, this systematic review and meta-analysis suggest a similar clinical profile for both continuation and interruption of OAC in patients undergoing TAVI, with continuation potentially offering a significant reduction in stroke risk, consistent with the published literature. However, our findings have certain limitations. First, the limited number of studies restricts the depth of subgroup analysis and the ability to analyze the underlying indication for anticoagulation (e.g., atrial fibrillation with varying CHA2DS2-VASc scores) and bleeding risk (e.g., the presence of peripheral arterial disease), which could affect the risk/benefit of continuing or interrupting anticoagulation during the TAVI, yet randomization assecured the balanced presence of atrial fibrillation, hypertension, and diabetes in the RCT. Additionally, the type of OAC used in the studies represents another important limitation. In observational studies, many patients on continued anticoagulation were treated with DOACs, while in the RCT, the use of VKAs was minimally represented, which may influence the applicability of the findings. Concerning the observational studies, only previous myocardial infarction history at baseline was different between groups, and only one study had a significant baseline difference for the presence of diabetes mellitus [6]. CHA2DS2-VASc scores were paired between all studies.
Second, although the number of studies available for inclusion was small, the patient sample of around 3000 was substantial for most outcomes. Third, variations in patient populations across individual studies may have contributed to the high heterogeneity in any or major bleeding. Additionally, non-standardized approaches in clinical practice have made it difficult to categorize patients for research purposes uniformly. Fourth, all studies reported only 30-day outcomes, leaving uncertainty about whether the benefits and risks of continued anticoagulation persist beyond this period. The lack of long-term follow-up limits our ability to assess sustained effects in TAVI patients. Fifth, although adjustments for multiple comparisons were not applied, the possibility of type I error should be acknowledged. Although these limitations may restrict the robustness of the conclusions, this meta-analysis provides important insights into the current evidence on OAC continuation versus interruption in patients undergoing TAVI. Further RCTs are needed to better understand the implications in specific subgroups, such as those at high risk of bleeding or stroke, and to eliminate confounding effects.
5 Conclusion
Our meta-analysis compared continuation versus interruption of OAC in patients undergoing TAVI with an indication for anticoagulation. Uninterrupted OAC was associated with fatal and hemorrhagic outcomes similar to those observed with interrupted OAC. Our analysis revealed a significant reduction in stroke risk. These results suggest that maintaining OAC during TAVI may be a viable and safe strategy for preventing cerebrovascular events, particularly in high-risk populations, without a marked increase in bleeding risk. However, the small number of included studies, modest sample sizes, and variability in patient populations underscore the need for further high-quality randomized trials to confirm these findings. Future research should also clarify which patients benefit most from uninterrupted OAC, helping establish more precise guidelines for perioperative anticoagulation management in this complex population.
Ethics Statement
The authors have nothing to report.
Consent
The authors confirm that patient consent is not applicable to this article. This is a systematic review and meta-analysis using publicly available, de-identified data; therefore, patient consent was not required.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




