Paclitaxel-Coated Balloons for Femoropopliteal Artery Disease Treatment in French Patients: A Longitudinal Observational Study
ABSTRACT
Background
Clinical studies have demonstrated the safety and effectiveness of drug-coated balloon (DCB) angioplasty for femoropopliteal revascularization. Long-term studies in routine practice are limited.
Aims
The aim of this study was to assess long-term outcomes after DCB angioplasty for femoropopliteal peripheral artery disease in a real-word-French population.
Methods
Patients with lower-limb PAD treated with at least one IN.PACT Admiral DCB in the year 2018 were identified from the French National Health Data System, representing > 99% of the French population. Primary outcomes were all-cause mortality, major amputation (including target and nontarget limbs and vessels), and reintervention (any infrainguinal reintervention or new intervention of the target lesions, nontarget lesions, target limbs, or contralateral limbs) within 1 year from the date of angioplasty. Patients were followed for 3 years from the date of angioplasty.
Results
A total of 3595 patients (average age 71.2 years) were enrolled, including 35.7% females, 35.3% with chronic limb-threatening ischemia (CLTI), 38.2% with diabetes, and 35.9% with a history of revascularization. All-cause mortality was 7.5% at 1 year and 19.7% at 3 years. The major amputation rate was 2.7% at 1 year and 4.6% at 3 years. The total reintervention rate was 25.9% at 1 year and 43.4% at 3 years. Three-year rates were significantly higher in patients with CLTI (vs. patients with intermittent claudication), diabetes (vs. no diabetes), and prior revascularization (vs. no prior revascularization).
Conclusions
This real-world analysis with long-term follow-up showed satisfactory limb salvage and low mortality following angioplasty with DCBs for the treatment of femoropopliteal artery disease.
Abbreviations
-
- CLTI
-
- critical limb-threatening ischemia
-
- DCB
-
- drug-coated balloon
-
- DCIR
-
- Données de Consommation Inter-Régime
-
- ICD
-
- International Classification of Disease
-
- PAD
-
- peripheral arterial disease
-
- PMSI
-
- Programme de Médicalisation des Systèmes d'Information
-
- SNDS
-
- Système National des Données de Santé
1 Introduction
Lower-limb peripheral arterial disease (PAD) is an increasingly serious public health problem affecting an estimated 236 million people worldwide [1]. Occlusive or stenotic lesions of the femoropopliteal artery are often treated by bypass graft or percutaneous transluminal angioplasty with or without placement of drug-coated balloons (DCB). Randomized controlled trials and prospective single-arm global registries have demonstrated the safety and effectiveness of DCB use in femoropopliteal lesions through 5 years of follow-up [2-8], leading to widespread dissemination of DCBs in the femoropopliteal vessels [9]. The long-term safety of paclitaxel-coated devices is also supported by a patient-level meta-analysis of devices approved by the US Food and Drug Administration [10, 11]. However, more evidence is needed on DCB use in national real-world settings, particularly in patients with poorer prognosis due to critical limb-threatening ischemia (CLTI) [12], comorbid diabetes mellitus [13], or a history of revascularization [14].
Nationwide healthcare databases in countries with nationalized healthcare services are valuable resources for exploring long-term outcomes after surgical interventions in routine clinical practice. We conducted a longitudinal assessment of all-cause mortality, major amputation, and reintervention after angioplasty with a DCB for the treatment of femoropopliteal PAD in a real-world setting using a French nationwide healthcare claims database.
2 Methods
2.1 Study Design
We conducted a prospective, protocol-driven, longitudinal, observational study of patients who underwent angioplasty with the IN.PACT Admiral paclitaxel DCB (Medtronic, Santa Rosa, California, USA) in France in 2018. Data were captured in the French National Health Data System (Système National des Données de Santé [SNDS]), which is the French nationwide claims database containing healthcare reimbursement data from patients affiliated with one of the compulsory health insurance providers representing more than 99% of French residents, including more than 10 years of follow-up. The use of deidentified SNDS data for this analysis was approved by the Ethics and Scientific Committee for Research, Studies and Evaluations in the Field of Health and Commission Nationale de l'Informatique et des Libertés.
SNDS contains data from three main sources: Système National d'Information Inter-Régimes de l'Assurance Maladie, which consists of all outpatient reimbursed health expenditures (Données de Consommation Inter-Régime [DCIR]), medical and administrative data from hospital stays (Programme de Médicalisation des Systèmes d'Information [PMSI]), and cause of death. Data from DCIR and PMSI have been linked for each patient to allow for follow-up across different settings of care, including the following: outpatient practices and hospital admissions related to medicine, surgery, and obstetrics; follow-up and rehabilitation care; and hospitalization at home. Each patient's healthcare use is tracked from birth or immigration in France to death or emigration, even if the person is not working, changes occupation, or retires, and irrespective of socioeconomic status.
SNDS contains information on beneficiaries' age, sex, region of residence, death date, complementary universal health coverage status, long-term diseases, and all outpatient healthcare consumption including all reimbursed prescription drugs identified by their Anatomical Therapeutic Chemical code, the date of delivery, quantity, and brand name. Medical procedures performed on an outpatient basis or in a healthcare institution are identified by the classification commune des actes médicaux (common classification of medical procedures), laboratory procedures are identified by the nomenclature des actes de biologie médicale (clinical pathology test nomenclature), and paramedical or medical visits are identified by a prestation code specific to the DCIR (PRS_NAT_REF). Codes of interest are detailed in Supporting Information S1: Table 1.
Through the PMSI, the SNDS also includes medical summaries of all hospitalizations from all private or public hospitals, including the date of stay, medical procedures, innovative and costly medications and medical devices covered by an add-on list (“Liste en sus”), the primary diagnosis (main reason for admission), related diagnoses (specifies the disease context of the primary diagnosis), and diagnoses related to other comorbidities, all encoded according to the International Classification of Disease, 10th edition (ICD-10). For certain medical devices (notably those covered by the “Liste en sus”), the type and brand of devices used are also tracked and reported.
For this analysis, we utilized SNDS data from the DCIR and the PMSI hospitalization data; specifically, data from hospitalizations in the medicine, surgery, and obstetrics sectors.
2.2 Study Population
This study comprised all adult patients with at least one reimbursement in SNDS for an IN.PACT Admiral DCB between January 1, 2018, and December 31, 2018. The study index date was the first hospital entry date for an angioplasty performed during the inclusion period. Data extraction began on January 1, 2015 and ended on December 31, 2021 to allow for (1) a 3-year historical period before the index procedure to identify patient comorbidities and any history of revascularization, and (2) a 3-year follow-up period from the date of angioplasty.
Patients included in the analysis had treatment for lower limb PAD. Patients who underwent angioplasty within 30 days before or 30 days after the index procedure were excluded from the analysis, as these were considered likely to be scheduled interventions (i.e., bilateral procedures) rather than interventions for restenosis. Because SNDS does not capture details of specific lesions, it was not possible to accurately identify the lesion characteristics.
2.3 Study Outcomes
The primary outcomes of this analysis were all-cause mortality, major amputation of a lower limb (including target and nontarget limbs and vessels), and rate of reintervention (any infrainguinal reintervention or new intervention of the target lesions, nontarget lesions, target limbs, or contralateral limbs performed through endovascular or surgical procedures) within 1 year after the index procedure.
Outcomes after the index procedure are described for the total study population and according to three clinical subgroups of interest: CLTI versus claudication, diabetes versus no diabetes, and history of revascularization versus no history of revascularization. Since there is no diagnostic code for CLTI, patients were defined as having CLTI if at least one diagnostic code associated with the presence of trophic disorders (ICD-10 code L97–lower limb ulcer, not elsewhere classified) was entered during the index stay, or if the index stay lasted more than two nights. By default, patients who did not meet any of the above criteria were considered to have claudication. Presence of diabetes was defined according to the Caisse Nationale de l'Assurance Maladie des Travailleurs Salariés algorithm, which identifies diabetes via hospitalizations for diabetes or for one of its complications, diabetes registered as a long-term disease, or at least three prescriptions of oral or injectable antidiabetic drugs (on three different dates) in the year preceding the index procedure [15]. The history of revascularization on the lower limb was defined as a lower limb intervention (regardless of laterality of the lesion) recorded in the database between 30 days and 2 years before the index procedure.
The SNDS database tracks any new procedure performed after the index procedure; however, the reason for and location of the new procedure (i.e., new lesion, reintervention, or bilateral intervention) are not captured. Similarly, deployment of stents during the index procedure is captured in the database with a procedure code for angioplasty with stenting, but the type and location of the stent may not be identified. Patients with one or more procedures at the infrainguinal level and one or more stents, without procedures identified at the iliac level, were considered to have had stent use during the index procedure, as well as patients for whom multi-leveled procedures and multiple stents were identified, without evidence of the location of stenting (Supporting Information S1: Figure 1).
2.4 Statistical Analysis
Event rates for primary outcomes (all-cause mortality, major amputation rate, and revascularization rate at 1 year) were estimated for all patients who had angioplasty with the IN.PACT Admiral DCB in 2018 using Kaplan−Meier analyses. Event rates at 1 year and 3 years after the index procedure were also described within the predefined subgroups (CLTI vs. claudication, diabetes vs. no diabetes, and history vs. no history of revascularization) and compared using the log-rank test. Kaplan−Meier curves describing freedom from all-cause mortality, major amputation, and revascularization over the 3-year follow-up were also plotted with their confidence intervals (CIs). Patients were censored on the date of death, the date of last reimbursement for care (in case of no benefit for a period of at least 10 months), or 3 years after inclusion, whichever occurred first. Statistical analyses were carried out by IQVIA (La Défense Cedex France) using SAS version 9.3 (SAS Institute, North Carolina, USA).
3 Results
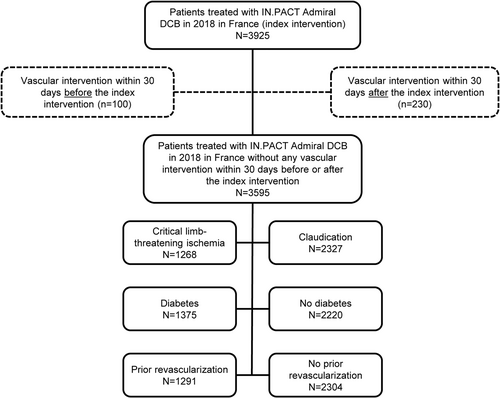
Between January 1, 2018, and December 31, 2018, 3925 patients in France underwent angioplasty with the IN.PACT Admiral DCB (i.e., the index procedure), of which 330 patients were excluded due to the presence of another vascular intervention within 30 days before or after the index procedure (Figure 1). The final study population of 3595 patients was 35.7% female with an average age of 71.2 ± 11.8 years (Table 1). CLTI was present in 35.3% of patients (N = 1268), diabetes was present in 38.2% of patients (N = 1375), and 35.9% of patients (N = 1291) had a history of revascularization. Most patients (74.3%) were prescribed aspirin and/or clopidogrel in the year before the index procedure; statin use was evident in 59.4% and angiotensin inhibitor agents in approximately one-third of patients. A mean of 1.2 ± 0.6 angioplasty procedures were performed, a mean of 1.3 ± 0.6 DCBs were used, and stents were deployed in 43.4% of patients (n = 1562), including any lower limb stenting (any vessel in either limb).
| Characteristics | Patients treated with IN.PACT Admiral DCB (N = 3595) |
|---|---|
| Demographics and patient comorbidities | |
| Women | 1285 (35.7) |
| Age (years) | 71.2 ± 11.8 |
| Critical limb-threatening ischemiaa | 1268 (35.3) |
| Smokingb | 1413 (39.3) |
| Diabetes | 1375 (38.2) |
| Treated with insulin | 622 (45.2) |
| Not treated with insulin | 753 (54.8) |
| Hypertension | 1946 (54.1) |
| Ischemic heart disease | 1230 (34.2) |
| End-stage renal failure | 419 (11.7) |
| Patients with a history of revascularizationc | 1291 (35.9) |
| Adjunctive medical therapiesd | |
| Statins | 2135 (59.4) |
| ACE Inhibitors | 1220 (33.9) |
| Angiotensin II receptor blockers | 1015 (28.2) |
| Aspirin and/or clopidogrel | 2670 (74.3) |
| Index procedure | |
| Length of stay (days) | 3.9 ± 6.96 |
| Number of angioplasties during index stay | 1.2 ± 0.55 |
| IN.PACT Admiral devices usede | 1.3 ± 0.62 |
| Stenting used during index proceduref | 1562 (43.4) |
- Note: Data are presented as n (%) or mean ± standard deviation.
- Abbreviation: ACE, angiotensin-converting enzyme.
- a Defined as at least one diagnostic code associated with a lower limb ulcer during the index stay or length of stay > 2 nights.
- b Smoking has been assessed via hospitalizations for smoking, associated diseases, COPD-specific treatments, and nicotine treatments.
- c History of revascularization on the lower limb was assessed in the period from 30 days to 2 years before angioplasty with the IN.PACT Admiral device.
- d Identified as at least three deliveries on three separate dates within 1 year before the index procedure date.
- e Number of IN.PACT Admiral devices registered during the index stay.
- f Includes all lower limb stenting (any vessel, any limb) and indeterminate stenting.
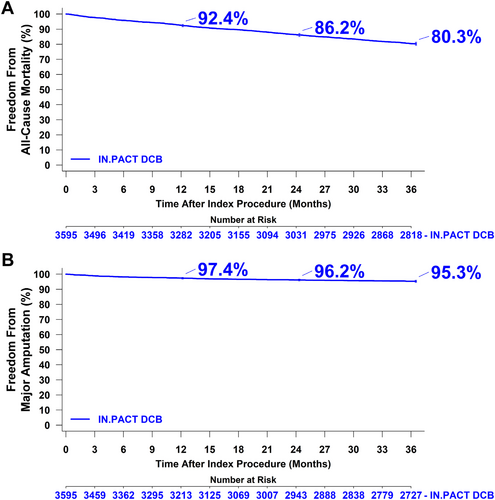
In the overall population at 1 year post-procedure, all-cause mortality was 7.5% (n = 271), the major amputation rate was 2.7% (n = 92), and the total reintervention rate was 25.9% (n = 896). At 1 year, the total reintervention rate included 22.5% (n = 777) endovascular reinterventions and 7.0% (n = 242) surgical reinterventions in any vessel, including the contralateral limb. At 3 years post-procedure, all-cause mortality was 19.7% (n = 698), the major amputation rate was 4.6% (n = 154), and the total reintervention rate was 43.4% (n = 1446), including 39.2% (n = 1298) endovascular reinterventions and 13.5% (n = 442) surgical reinterventions in any vessel/limb. Overall survival was 92.4% at 1 year, 86.2% at 2 years, and 80.3% at 3 years post-procedure (Figure 2A). The rates of freedom from major amputation were 97.4% at 1 year, 96.2% at 2 years, and 95.3% at 3 years post-procedure (Figure 2B).
Patients with CLTI were slightly older, more likely to be female, and more likely to have diabetes, hypertension, and end-stage renal failure than patients with claudication (Table 2). At 3 years post-procedure, all-cause mortality (30.6% vs. 13.9%, p < 0.001), the major amputation rate (9.5% vs. 2.3%, p < 0.001), and the total reintervention rate (50.8% vs. 39.8%, p < 0.001) were significantly higher among patients with CLTI than those with claudication. Both the endovascular revascularization rate (44.6% vs. 36.5%, p < 0.001) and the open surgery revascularization rate (19.9% vs. 10.4%, p < 0.001) were also higher among patients with CLTI. Rates of freedom from all-cause mortality and freedom from major amputation at 3 years post-procedure were significantly lower for patients with CLTI (Figure 3A).
Critical limb-threatening ischemiaa (N = 1268) |
Claudication (N = 2327) | Diabetes (N = 1375) | No diabetes (N = 2220) | Prior revasc-ularizationc (N = 1291) | No prior revasc-ularization (N = 2304) | |
|---|---|---|---|---|---|---|
| Demographics and patient comorbidities | ||||||
| Women | 520 (41.0) | 765 (32.9) | 428 (31.1) | 857 (38.6) | 471 (36.5) | 814 (35.3) |
| Age (years) | 73.7 ± 12.2 | 69.8 ± 11.3 | 71.4 ± 10.7 | 71.0 ± 12.4 | 71.6 ± 11.5 | 70.9 ± 11.9 |
| Critical limb-threatening ischemiaa | ― | ― | 552 (40.1) | 716 (32.3) | 470 (36.4) | 798 (34.6) |
| Smokingb | 482 (38.0) | 931 (40.0) | 505 (36.7) | 908 (40.9) | 528 (40.9) | 885 (38.4) |
| Diabetes | 552 (43.5) | 823 (35.4) | ― | ― | 543 (42.1) | 832 (36.1) |
| Treated with insulin | 268 (48.6) | 354 (43.0) | 622 (45.2) | ― | 276 (50.8) | 346 (41.6) |
| Not treated with insulin | 284 (51.4) | 469 (57.0) | 753 (54.8) | ― | 267 (49.2) | 486 (58.4) |
| Hypertension | 822 (64.8) | 1124 (48.3) | 873 (63.5) | 1073 (48.3) | 778 (60.3) | 1168 (50.7) |
| Ischemic heart disease | 461 (36.4) | 769 (33.0) | 570 (41.5) | 660 (29.7) | 488 (37.8) | 742 (32.2) |
| End-stage renal failure | 201 (15.9) | 218 (9.4) | 250 (18.2) | 169 (7.6) | 177 (13.7) | 242 (10.5) |
| Patients with a history of revascularizationc | 470 (37.1) | 821 (35.3) | 543 (39.5) | 748 (33.7) | ― | ― |
| Adjunctive medical therapiesd | ||||||
| Statins | 697 (55.0) | 1438 (61.8) | 927 (67.4) | 1,208 (54.4) | 839 (65.0) | 1296 (56.3) |
| ACE inhibitors | 423 (33.4) | 797 (34.3) | 552 (40.1) | 668 (30.1) | 465 (36.0) | 755 (32.8) |
| Angiotensin II receptor blocker | 361 (28.5) | 654 (28.1) | 455 (33.1) | 560 (25.2) | 365 (28.3) | 650 (28.2) |
| Aspirin and/or clopidogrel | 894 (70.5) | 1,776 (76.3) | 1074 (78.1) | 1596 (71.9) | 1065 (82.5) | 1605 (69.7) |
| Index procedure | ||||||
| Length of stay (days) | 8.4 ± 10.26 | 1.5 ± 0.71 | 4.8 ± 8.34 | 3.4 ± 5.89 | 4.1 ± 7.41 | 3.9 ± 6.70 |
| Number of angioplasties during index stay | 1.3 ± 0.63 | 1.2 ± 0.49 | 1.2 ± 0.57 | 1.2 ± 0.53 | 1.2 ± 0.55 | 1.2 ± 0.55 |
| IN.PACT Admiral devices usede | 1.3 ± 0.68 | 1.3 ± 0.59 | 1.3 ± 0.64 | 1.3 ± 0.61 | 1.3 ± 0.65 | 1.3 ± 0.60 |
| Stenting used during index proceduref | 578 (45.6) | 984 (42.3) | 566 (41.2) | 996 (44.9) | 476 (36.9) | 1086 (47.1) |
- Note: Data are presented as n (%) or mean ± standard deviation.
- Abbreviation: ACE, angiotensin-converting enzyme.
- a Defined as at least one diagnostic code associated with a lower limb ulcer during the index stay or length of stay > 2 nights.
- b Smoking has been assessed via hospitalizations for smoking, associated diseases, COPD-specific treatments, and nicotine treatments.
- c History of revascularization on the lower limb was assessed in the period from 30 days to 2 years before angioplasty with the IN.PACT Admiral device.
- d Identified as at least three deliveries on three separate dates within 1 year before the index procedure date.
- e Number of IN.PACT Admiral devices registered during the index stay.
- f Includes all lower limb stenting (any vessel, any limb) and indeterminate stenting.
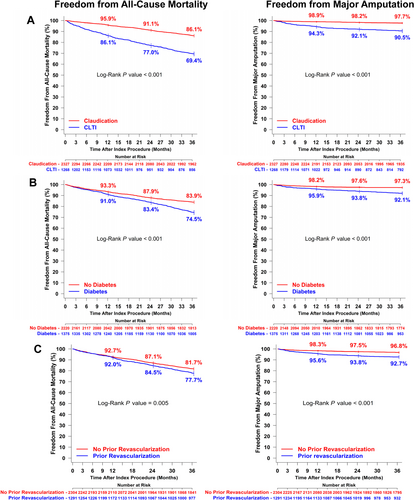
Patients with diabetes were more likely to be male and more likely to have CLTI, hypertension, ischemic heart disease, end-stage renal failure, and a history of revascularization than those without diabetes (Table 2). At 3 years post-procedure, all-cause mortality (25.5% vs. 16.1%, p < 0.001), the major amputation rate (7.9% vs. 2.5%, p < 0.001), and the total reintervention rate (48.1% vs. 40.6%, p < 0.001) were higher among patients with diabetes than those without diabetes. The endovascular revascularization rate (44.9% vs. 35.7%, p < 0.001) was also higher among patients with diabetes but open surgery revascularization rates did not differ between those with and without diabetes (12.3% and 14.0%, p = 0.25). Rates of freedom from all-cause mortality and freedom from major amputation at 3 years post-procedure were significantly lower for patients with diabetes compared to those without diabetes (Figure 3B).
Patients with a history of revascularization were more likely to have diabetes, hypertension, and end-stage renal failure than those without a history of revascularization (Table 2). At 3 years post-procedure, all-cause mortality (22.3% vs. 18.3%, p = 0.005), the major amputation rate (7.3% vs. 3.2%, p < 0.001), and the total reintervention rate (51.7% vs. 38.9%, p < 0.001) were higher among patients with a history of revascularization than those without. Both the endovascular revascularization rate (47.1% vs. 34.8%, p < 0.001) and the open surgery revascularization rate (17.5% vs. 11.3%, p < 0.001) were higher among patients with a history of revascularization. Rates of freedom from all-cause mortality and freedom from major amputation were significantly lower at 3 years post-procedure for patients with a history of revascularization compared to those with no history of revascularization (Figure 3C).
4 Discussion
This observational study followed more than 3500 patients for 3 years after undergoing angioplasty with a paclitaxel-coated DCB in the femoropopliteal artery. Patients enrolled were typical of a real-world PAD population. At 1- and 3-year follow-up, respectively, 2.7% and 4.6% of patients had a major amputation of the target or nontarget limb, 7.5% and 19.7% had died due to any cause, and the infrainguinal reintervention rate (any vessel in either limb) was 25.9% and 43.4%. These all-cause mortality rates at 1 and 3 years are comparable to prior clinical trials and registry studies of the IN.PACT Admiral DCB [16-21].
Rates of major amputation and reintervention were higher than observed in prior studies, owing to the fact that the SNDS database does not specify whether amputations and reinterventions were of the target limb or vessel. Contralateral reintervention rates of 20%−30% have been reported after infrainguinal bypass or percutaneous revascularization in PAD patients [22, 23]. Furthermore, prior IN.PACT studies evaluated primarily patients with intermittent claudication or rest pain (Rutherford classification 2−4) associated with an ischemic femoropopliteal lesion. In our study of routine clinical practice, patients with minor tissue damage and/or ulceration and gangrene (Rutherford classification 5−6) were eligible, and 35% of patients were identified with CLTI.
Finally, use of provisional stenting at the index procedure (43.4% of lesions) in our study was higher than in the varied geographic regions represented in prior studies (IN.PACT Global, 21.2%; DES Italian Registry, 12.3%; IN.PACT SFA study, 7.3%; and IN.PACT SFA Japan, 4.4%) [17-20], and included all lower limb stenting (any vessel, any limb) unlike prior studies that focused on the target lesion.
4.1 Limb Salvage
Our 3-year major amputation rate of 4.6% in routine clinical practice is low given the unselected population, high proportion of patients with CLTI, and the fact that this figure may include amputation of the nontarget limb. Three-year target limb amputation rates in the IN.PACT studies were very low (IN.PACT Global, 1.0%; IN.PACT SFA, 0%; and IN.PACT SFA Japan, 0%) [18-20]. Together, these findings demonstrate successful limb salvage with this DCB.
4.2 Mortality
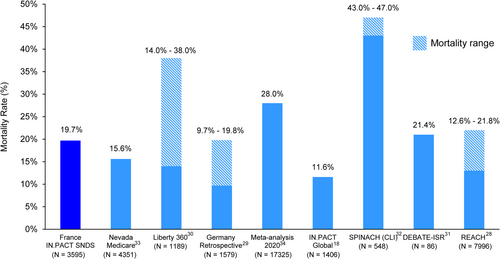
A recently updated meta-analysis of mortality rates following the use of paclitaxel-coated interventional devices for the treatment of lower extremity PAD supports the safe use of paclitaxel DCBs in randomized trial settings [10]. The current large, national registry further supports the use of paclitaxel-coated devices in real-world settings. The 1-year (7.5%) mortality rates in our study compare favorably with rates in other real-world evaluations of patients with PAD, including the COPART Registry in France (21.4%) [24] and a Canadian analysis (8.2% annual mortality) [25], as well as in real-world studies of paclitaxel-coated device use in Sweden (10.2%) [26] and the United States (8.6%) [27]. Three-year mortality rates vary widely across real-world PAD studies (9.7%−47.0%) and the rate of 19.7% in the current study falls in the middle of that range, consistent with half of the previous studies (Figure 4) [18, 28-34].
4.3 High-Risk Subgroups
CLTI is the most severe manifestation of PAD and presents with ischemic pain at rest, tissue loss, or both. In post hoc analysis of the 5-year follow-up in the IN.PACT Global study, participants with CLTI had significantly higher cumulative incidence of all-cause mortality (37.4% vs. 17.4%; p < 0.001) and major target limb amputation (6.8% vs. 1.1%; p < 0.001) compared to participants with intermittent claudication [35]. Consistent with these findings, we observed significantly higher rates of all-cause mortality (30.6% vs. 13.9%, p < 0.001), major amputation (9.5% vs. 2.3%, p < 0.001), and total reintervention (50.8% vs. 39.8%, p < 0.001) in patients with CLTI compared to patients with intermittent claudication at 3 years. Our findings for patients with CLTI are also consistent with rates of all-cause mortality (33.0% for surgery and 37.6% for endovascular therapy) and above-the-knee amputation (10.4% and 14.9%, respectively) at median 2.7 years follow-up in the BEST-CLI randomized trial of patients with CLTI and infrainguinal PAD with a single segment of the great saphenous vein that could be used for surgery [36]. However, higher rates of all-cause mortality (53% for vein bypass and 45% for endovascular treatment) and major amputation (20% and 18%, respectively) at 2-year follow-up have been reported in the BASIL-2 randomized, multicenter trial in patients with CLTI [37].
The known increased mortality risk for PAD patients with comorbid diabetes mellitus [38] was not observed in the 3-year analysis of IN.PACT Global [18] but was evident in the post hoc analysis of 5-year follow-up, in which only all-cause mortality was higher in patients with diabetes mellitus compared to those without diabetes (23.8% vs. 16.6%; p < 0.001) [35]. In our real-world study, not only was the 3-year rate of all-cause mortality (25.5% vs. 16.1%, p < 0.001) significantly higher among patients with diabetes than those without diabetes, but the same was true for major amputation (7.9% vs. 2.5%, p < 0.001), and total reintervention (48.1% vs. 40.6%, p < 0.001).
Patients with PAD who have undergone a prior revascularization procedure carry a particularly high risk for additional adverse limb events. In the IN.PACT global study, a history of prior revascularization was present in 52% of patients and was a significant multivariable predictor of major adverse events [3]. Previous ipsilateral revascularization was also identified as a predictor of clinically driven target lesion revascularization among women in IN.PACT SFA randomized trial [39]. Corroborating these results in a real-world French population, our study observed that a history of revascularization was associated with poorer outcomes, including all-cause mortality (22.3% vs. 18.3%, p = 0.005), major amputation (7.3% vs. 3.2%, p < 0.001), and total reintervention (51.7% vs. 38.9%, p < 0.001), compared to those without prior revascularization.
4.4 Study Limitations
This was a study of real-world experience with a DCB in femoropopliteal artery disease using data from the French national healthcare claims database. It was a single-arm retrospective analysis with no comparator group and no adjudication of outcomes. The SNDS database does not contain detailed lesion characteristics or reintervention details, such as vessel location or side; thus, major amputations and interventions could be in any vessel or the contralateral limb, resulting in overestimations compared to other prospective registries. These different definitions of major amputation and reintervention used in our study preclude direct comparison of these rates with other studies of DCBs. The lack of a diagnostic code for CLTI and the necessary use of a surrogate measure was also a limitation. Nonetheless, this longitudinal, prospective national study with near-total coverage in France is a useful tool for confirming or identifying potential differences in outcomes between clinical trials and routine practice.
5 Conclusions
Analysis of a nationwide healthcare claims database showed satisfactory limb salvage and low mortality following angioplasty with a paclitaxel-coated DCB for the treatment of PAD. Real-world analyses of this kind are essential for continued monitoring of the post-market performance of DCBs for revascularization of femoropopliteal artery disease.
Acknowledgments
This work was funded by Medtronic. The authors thank the following employees of IQVIA France for their support: Emilie Casarotto (study design and analysis of 1-year data), Soline Leblanc (data analysis), and Pierre Lemire (supervision of 3-year data analysis). The authors also thank Fanny Wilquin-Bequet, Stefanie Deckers, and Kristin Hood, PhD of Medtronic for project management support, Jeremiah Menk of Medtronic for creating the Kaplan−Meier figures, and Laurie LaRusso, MS ELS (Chestnut Medical Communications) for medical writing support funded by Medtronic.
Conflicts of Interest
Y.G. reports consulting agreements with Boston Scientific Corporation, Cook Medical, Medtronic, and Perouse Medical and institutional grant/research support from Medtronic, St. Jude Medical, and Terumo Interventional Systems. A.T. is a full-time employee of IQVIA Operations France, which was funded by Medtronic to conduct the analysis. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




