PCSK-9 Inhibitors Can Significantly Improve the Coronary Slow Flow Caused by Elevated Lipoprotein (a) in ST-Elevation Myocardial Infarction Patients With Chronic Kidney Disease
Hao Xu and Ziqing Wang contributed equally to this study and considered co-first authors.
ABSTRACT
Background
Coronary slow flow and no reflow significantly predict poor prognosis in acute myocardial infarction (AMI) patients, especially those with chronic kidney disease (CKD). Early identification of factors contributing to these conditions can mitigate ischemic events and improve outcomes.
Aims
This study aimed to investigate the association between elevated lipoprotein (a) [Lp(a)] levels and proprotein convertase subtilisin/kexin Type 9 (PCSK-9) inhibitor therapy with coronary slow flow or no reflow after percutaneous coronary intervention (PCI) in AMI patients with CKD.
Methods
A total of 323 ST-elevation myocardial infarction (STEMI) patients who underwent PCI between October 2017 and June 2023 were included. Patients were divided into CKD (n = 132) and non-CKD (n = 191) groups. Lp(a) levels and the prevalence of coronary slow flow or no reflow after PCI were evaluated. STEMI patients with CKD were further categorized into elevated Lp(a) (n = 81) and normal Lp(a) (n = 51) subgroups. Logistic analysis identified risk factors for coronary slow flow/no reflow after PCI. The impact of PCSK-9 inhibitors on outcomes was also assessed in the elevated Lp(a) subgroup.
Results
STEMI patients with CKD had significantly higher Lp(a) levels compared to those without CKD (median 36.75 vs. 15.90 mg/dL, p = 0.0001). CKD patients with elevated Lp(a) had a higher prevalence of coronary slow flow/no reflow after PCI than those with normal Lp(a) (38.3% vs. 13.7%, p = 0.002). Logistic regression analysis identified elevated Lp(a) as an independent risk factor for slow flow/no reflow after PCI in STEMI patients with CKD (OR = 2.985, p = 0.027). In CKD patients with elevated Lp(a), PCSK-9 inhibitors significantly improved post-PCI coronary flow and reduced composite cardiovascular events during 1-year follow-up (22.2% vs. 51.1%, p = 0.008).
Conclusions
Elevated Lp(a) is an independent risk factor for coronary slow flow or no reflow after PCI in STEMI patients with CKD. PCSK-9 inhibitors improve coronary blood flow and reduce cardiovascular events in these patients.
Abbreviations
-
- ACS
-
- acute coronary syndrome
-
- AIP
-
- atherogenic index of plasma
-
- ALT
-
- glutamic-pyruvic transaminase
-
- AMI
-
- acute myocardial infarction
-
- AST
-
- glutamic oxalacetic transaminase
-
- BMI
-
- body mass index
-
- CAG
-
- coronary angiography
-
- CHD
-
- coronary heart disease
-
- CKD
-
- chronic kidney disease
-
- CRP
-
- C-reactive protein
-
- D-D
-
- D-dimer
-
- eGFR
-
- estimate glomerular filtration rate
-
- HDL-C
-
- high density lipoprotein cholesterol
-
- INR
-
- international normalized ratio
-
- LAD
-
- left anterior descending
-
- LCX
-
- left circumflex
-
- LDL-C
-
- low density lipoprotein cholesterol
-
- Lp(a)
-
- lipoprotein (a)
-
- MACCEs
-
- major adverse cardio-cerebrovascular events
-
- OR
-
- odds ratio
-
- PCI
-
- percutaneous coronary intervention
-
- PCSK-9
-
- proprotein convertase subtilisin/kexin type 9
-
- PT
-
- prothrombin activity
-
- PTCA
-
- percutaneous transluminal coronary angioplasty
-
- RCA
-
- right coronary artery
-
- SI
-
- shock index
-
- STEMI
-
- ST-elevation myocardial infarction
-
- TC
-
- total cholesterol
-
- TG
-
- triglyceride
1 Introduction
The prevalence of coronary heart disease (CHD) and chronic kidney disease (CKD) is rising, with CKD patients facing high mortality rates due to an increased risk of adverse cardiovascular outcomes. Kidney disease itself is an independent risk factor for these outcomes. Emerging evidence indicates that elevated Lp(a) levels not only contribute to cardiovascular diseases such as heart failure [1], aortic valve calcification [2], and coronary heart disease [3], but are also linked to declining kidney function [4].
Despite advancements in percutaneous coronary intervention (PCI) and the widespread use of adequate anticoagulation therapy, which have significantly reduced the incidence of the slow flow/no reflow phenomenon after PCI over recent decades, it remains a concern. Slow flow/no reflow, characterized by impaired forward flow in the epicardial coronary arteries after mechanical obstruction is removed, leading to myocardial hypoperfusion, still occurs in 10%–30% of patients with acute myocardial infarction (AMI) according to recent epidemiological studies, especially in ST-elevation myocardial infarction (STEMI) [5, 6]. Previous studies have identified several independent risk factors for coronary slow flow/no reflow in AMI patients, including preoperative blood glucose levels, diabetes [7], atherogenic index of plasma (AIP) [8], shock index (SI) [9], C-reactive protein (CRP) [10], and decreased renal function [11].
In patients with CKD, activation of the renin-angiotensin system and dyslipidemia increase oxidative stress, promote the expression of inflammatory mediators, damage endothelial cells, and contribute to coronary slow flow or no reflow [12, 13]. Previous studies have demonstrated that decreased renal function is significantly associated with elevated lipoprotein (a) [Lp(a)] levels, reduced coronary blood flow reserve, and slow coronary blood flow [12, 14, 15].
Contemporary evidence suggests that Lp(a) not only accelerates coronary atherosclerosis but also competes with plasminogen to bind fibrin sites, promoting thrombosis and leading to coronary slow flow or no reflow [16, 17]. While Lp(a) is implicated in these phenomena through multiple mechanisms, its precise relationship with coronary slow flow or no reflow remains unclear.
There is limited research on the relationship between Lp(a) levels and coronary slow flow or no reflow in CKD patients, particularly in those with STEMI. This study aims to explore the association of elevated Lp(a) levels and the effect of PCSK-9 inhibitor therapy on coronary slow flow or no reflow after PCI in STEMI patients with CKD.
2 Methods
2.1 Study Design and Population
This study was a single-center, retrospective case–control study conducted at our hospital. This study included 323 patients (aged 25–80 years) with STEMI who underwent PCI at our hospital between October 2017 and June 2023.
- 1.
Previously diagnosed CHD treated with PCI or percutaneous transluminal coronary angioplasty (PTCA);
- 2.
Major surgical procedures within the last year;
- 3.
Severe liver insufficiency;
- 4.
Malignant tumors;
- 5.
Diseases affecting lipid metabolism, such as nephrotic syndrome, systemic lupus erythematosus, pancreatitis, or thyroid disorders (hyperthyroidism or hypothyroidism);
- 6.
Age over 80 years.
2.2 Definitions and Medication Therapy
STEMI was defined according to the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guidelines for the Evaluation and Diagnosis of Chest Pain [18]. CKD was defined based on the National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease [19]. Slow flow was defined as blood flow to the distal end of the occluded vessel taking more than one cardiac cycle or failing to reach the distal vessel end. Nonreflow was defined as the complete disappearance of forward coronary flow after mechanical blockage of the coronary artery was relieved, with no residual stenosis, thrombosis, dissection, or spasm [20]. Renal dysfunction was classified based on estimated glomerular filtration rate (eGFR) as follows: mild renal dysfunction: eGFR ≥ 60 mL/min/1.73 m2; moderate renal dysfunction: 30 ≤ eGFR < 60 mL/min/1.73 m2; severe renal dysfunction: eGFR < 30 mL/min/1.73 m2. Drinking history was defined as an average daily alcohol intake greater than 40 g for men and 20 g for women, with a drinking duration of at least 5 years. Elevated Lp(a) levels were defined as > 30 mg/dL.
All patients received statin therapy (either 10 mg atorvastatin or 20 mg rosuvastatin). Patients who exhibited elevated Lp(a) levels (> 30 mg/dL) and failed to achieve LDL-C targets (> 1.4 mmol/L) were evaluated for eligibility for PCSK-9 inhibitor therapy on the first postoperative day. PCSK-9 inhibitor therapy was defined as therapy with either 140 mg evolocumab or 75 mg alirocumab, administered every 2 weeks for a minimum duration of least 6 months.
2.3 Grouping
Patients were divided into two groups: CKD (n = 132) and non-CKD (n = 191). Among patients with CKD, subgroups were defined based on Lp(a) levels: the elevated Lp(a) group (> 30 mg/dL, n = 81) and the normal Lp(a) group (≤ 30 mg/dL, n = 51). In the elevated Lp(a) group, 36 patients received PCSK-9 inhibitor therapy, while 45 did not.
2.4 Study Endpoints
In the subgroup elevated Lp(a) group, patients were followed for 1 year. The primary endpoints included bleeding events, worsening renal function, and major adverse cardio-cerebrovascular events (MACCEs), defined as cardiac death, nonfatal myocardial infarction, nonfatal stroke, readmission for angina, and readmission for heart failure.
2.5 Statistical Methods
Statistical analyses were performed using SPSS version 25.0 (IBM SPSS Statistics, Chicago, USA). Continuous data with a normal or approximately normal distribution were expressed as mean ± SD, and comparisons between groups were conducted using the T-test. For continuous data with a skewed distribution, results were expressed as the median (Q1, Q3), and group comparisons were performed using the Mann–Whitney test. One-way Analysis of variance (ANOVA) was used to assess statistically significant differences among the means of three independent groups. Categorical data were presented as frequencies (%), and comparisons between groups were carried out using the χ2 test. Univariate logistic regression analysis was conducted to identify potential predictors of slow flow or no reflow after PCI. Variables with p < 0.05 in the univariate analysis were included in the multivariate logistic regression model to determine independent predictors. Multicollinearity among independent variables was assessed using the variance inflation factor (VIF). A VIF threshold of 10 was used to identify severe multicollinearity. Given the high VIF values observed for total cholesterol (TC) in both the overall AMI cohort and the AMI subgroup with CKD, TC was excluded from the final multivariable regression models to ensure model stability. A two-sided test was applied for all analyses, with a significance level set at α = 0.05, and results were considered statistically significant when p < 0.05.
3 Results
3.1 Baseline Clinical Characteristics of STEMI Patients Underwent PCI
Baseline clinical characteristics are summarized in Table 1. Compared to the non-CKD group, the CKD group exhibited significantly higher levels of triglyceride (TG) (median 1.57 vs. 1.35 mmol/L, p = 0.022), Lp(a) (median 36.75 vs. 15.90 mg/dL, p = 0.0001), blood glucose (7.20 ± 2.81 vs. 6.27 ± 2.90 mmol/L, p = 0.004), and fibrinogen (3.73 ± 0.90 vs. 2.93 ± 0.71 g/L, p = 0.0001). In contrast, high density lipoprotein cholesterol (HDL-C) levels were significantly lower in the CKD group (1.16 ± 0.33 vs. 1.24 ± 0.32 mmol/L, p = 0.033). Low density lipoprotein cholesterol (LDL-C) levels were comparable between the two groups. The prevalence of coronary slow flow or no reflow after PCI was significantly higher in the CKD group compared to the non-CKD group (28.79% vs. 14.66%, p = 0.002).
| CKD group (n = 132) | non-CKD group (n = 191) | p value | |
|---|---|---|---|
| General information | |||
| Male | 82 (62.1%) | 119 (62.3%) | 0.973 |
| Age (years) | 63.97 ± 11.41 | 60.50 ± 11.18 | 0.007b |
| BMI (kg/m2) | 25.55 ± 3.58 | 25.90 ± 3.29 | 0.363 |
| Smoking history | 24 (18.2%) | 57 (29.8%) | 0.017b |
| Drinking history | 20 (15.2%) | 33 (17.3%) | 0.612 |
| Diabetes | 71 (53.8%) | 43 (22.5%) | 0.0001b |
| Hypertension | 105 (79.5%) | 105 (55.0%) | 0.0001b |
| Assay index | |||
| TC (mmol/L) | 4.61 ± 1.52 | 4.51 ± 1.21 | 0.531 |
| TG (mmol/L) | 1.57 (1.08, 2.19) | 1.35 (0.92, 1.84) | 0.022b |
| HDL-C (mmol/L) | 1.16 ± 0.33 | 1.24 ± 0.32 | 0.033b |
| LDL-C (mmol/L) | 2.76 ± 1.15 | 2.68 ± 1.00 | 0.489 |
| Lp(a) (mg/dL) | 36.75 (18.05, 71.4) | 15.90 (5.71, 34.95) | 0.0001b |
| ALT (U/L) | 21 (13.8, 30.8) | 27.7 (20.7, 46) | 0.0001b |
| AST (U/L) | 21.65 (15, 29.05) | 28 (20.85, 42) | 0.0001b |
| Blood glucose (mmol/L) | 7.20 ± 2.81 | 6.27 ± 2.90 | 0.004b |
| Potassium (mmol/L) | 4.42 ± 0.69 | 4.11 ± 0.44 | 0.0001b |
| Sodium (mmol/L) | 139.43 ± 3.94 | 139.98 ± 2.52 | 0.159 |
| Creatinine (µmol/L) | 144 (111, 238.95) | 845 (75.75, 95.9) | 0.0001b |
| eGFR (mL/min/1.73 m2) | 43.21 (51.53,56.89) | 74.73 (65.66, 87.25) | 0.0001b |
| INR | 1.02 ± 0.13 | 1.04 ± 0.11 | 0.415 |
| Fibrinogen (g/L) | 3.73 ± 0.90 | 2.93 ± 0.71 | 0.0001b |
| PT (%) | 99.09 ± 22.80 | 96.87 ± 15.71 | 0.334 |
| D-D (ng/mL) | 375 (280, 540) | 300 (200, 435) | 0.0001b |
| CAG result | |||
| Slow flow/no reflow | 38 (28.79%) | 28 (14.66%) | 0.002b |
| Slow-TIMI 3 | 26 (19.70%) | 17 (8.90%) | 0.005b |
| TIMI 2 | 8 (6.06%) | 9 (4.71%) | 0.594 |
| TIMI 1 | 3 (2.27%) | 0 | 0.133 |
| TIMI 0 | 1 (0.76%) | 2 (1.05%) | 1.000 |
- Abbreviations: ALT, glutamic-pyruvic transaminase; AST, glutamic oxalacetic transaminase; BMI, body mass index; CAG, coronary angiography; CKD, chronic kidney disease; D-D, D-dimer; eGFR, estimate glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; INR, international normalized ratio; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein (a); PCI, percutaneous coronary intervention; PT, prothrombin activity; TC, total cholesterol; TG, triglyceride
- a Data are presented as the mean ± SD, median (Q1, Q3), or n (%).
- b p < 0.05, indicating statistical significance.
3.2 Baseline Clinical Characteristics of PCI Patients With CKD Stratified by Lp(a) Levels
A total of 132 PCI patients with CKD were evaluated: 28 (21.21%) with mild, 62 (46.97%) with moderate, and 42 (31.82%) with severe renal dysfunction. Lp(a) levels increased with worsening renal function (median: 24.60 vs. 32.60 vs. 55.45 mg/dL, p = 0.002). Patients with CKD were accordingly divided into two groups: the elevated Lp(a) group (n = 81) and the normal Lp(a) group (n = 51). Baseline clinical characteristics of patients with CKD (n = 132) stratified by Lp(a) levels are presented in Table 2. No significant differences in age, smoking history, drinking history, hypertension, diabetes, or triglycerides were observed between the groups. At the same time, there was no difference between the two groups in the procedural characteristics of PCI surgery (time from onset of chest pain to culprit vessel patency, culprit vessel, stent mean diameter, total stent length, all p > 0.05). However, the elevated Lp(a) group had higher levels of total cholesterol (TC, median: 4.78 vs. 3.89 mmol/L, p = 0.0001), HDL-C (1.23 ± 0.35 vs. 1.04 ± 0.24 mmol/L, p = 0.0001), LDL-C (3.07 ± 1.24 vs. 2.27 ± 0.77 mmol/L, p = 0.0001), fibrinogen (3.93 ± 0.91 vs. 3.43 ± 0.81 g/L, p = 0.002), and prothrombin activity (PT, 102.42 ± 25.57% vs. 93.78 ± 16.41%, p = 0.019), along with shorter clotting time (INR, 1.01 ± 0.13 vs. 1.05 ± 0.11, p = 0.038). Coronary slow flow or no reflow after PCI were more common in the elevated Lp(a) group than in the normal Lp(a) group (38.27% vs. 13.73%, p = 0.002). Post-PCI blood flow grading was as follows: in the elevated Lp(a) group, TIMI3: 61.73%, slow-TIMI3: 27.16%, TIMI2: 7.41%, TIMI1: 2.47%, TIMI0: 1.23%; in the normal Lp(a) group, TIMI3: 86.27%, slow-TIMI3: 7.84%, TIMI2: 3.92%, TIMI1: 1.96%, TIMI0: 0%.
| Elevated Lp(a) group (n = 81) | Normal Lp(a) group (n = 51) | p value | |
|---|---|---|---|
| General information | |||
| Male | 44 (54.3%) | 38 (74.5%) | 0.020b |
| Age (years) | 63.25 ± 11.25 | 65.12 ± 11.69 | 0.361 |
| BMI (kg/m2) | 25.02 ± 3.30 | 26.38 ± 3.89 | 0.034b |
| Smoking history | 14 (17.3%) | 10 (19.6%) | 0.736 |
| Drinking history | 10 (12.3%) | 10 (19.6%) | 0.257 |
| Diabetes | 42 (51.9%) | 29 (56.9%) | 0.574 |
| Hypertension | 62 (76.5%) | 43 (84.3%) | 0.281 |
| Assay index | |||
| TC (mmol/L) | 4.78 (3.94, 5.46) | 3.89 (3.19, 4.66) | 0.0001b |
| TG (mmol/L) | 1.61 (1.15, 2.25) | 1.41 (0.95,1.91) | 0.087 |
| HDL-C (mmol/L) | 1.23 ± 0.35 | 1.04 ± 0.24 | 0.0001b |
| LDL-C (mmol/L) | 3.07 ± 1.24 | 2.27 ± 0.77 | 0.0001b |
| Lp(a) (mg/dL) | 61.1 (42.6, 102.5) | 13.1 (7.65, 21.05) | 0.0001b |
| ALT (U/L) | 20.6 (13.8, 28) | 23.2 (14.4, 38.35) | 0.202 |
| AST (U/L) | 19.5 (15, 27) | 22.7 (16.55,32.5) | 0.161 |
| Blood glucose (mmol/L) | 7.06 ± 2.78 | 7.42 ± 2.87 | 0.472 |
| Potassium (mmol/L) | 4.52 ± 0.74 | 4.27 ± 0.58 | 0.046b |
| Sodium (mmol/L) | 139.54 ± 4.21 | 139.25 ± 3.49 | 0.688 |
| INR | 1.01 ± 0.13 | 1.05 ± 0.11 | 0.038b |
| Fibrinogen (g/L) | 3.93 ± 0.91 | 3.43 ± 0.81 | 0.002b |
| PT (%) | 102.42 ± 25.57 | 93.78 ± 16.41 | 0.019b |
| D-D (ng/mL) | 380 (290, 540) | 370 (280, 500) | 0.596 |
| CAG result | |||
| Slow flow/no reflow | 31 (38.27%) | 7 (13.73%) | 0.002b |
| Slow-TIMI 3 | 22 (27.16%) | 4 (7.84%) | 0.007b |
| TIMI 2 | 6 (7.41%) | 2 (3.92%) | 0.658 |
| TIMI 1 | 2 (2.47%) | 1 (1.96%) | 1.000 |
| TIMI 0 | 1 (1.23%) | 0 | 1.000 |
| Time (h)c | 7.00 ± 2.31 | 6.81 ± 2.45 | 0.653 |
| Mean diameter of stent (mm) | 3.19 ± 0.42 | 3.31 ± 0.43 | 0.114 |
| Total length of bracket (mm) | 29.25 ± 8.68 | 27.55 ± 8.44 | 0.271 |
| Convict vessel | |||
| LAD | 30 (37.0%) | 22 (43.1%) | 0.485 |
| LCX | 12 (14.8%) | 9 (17.6%) | 0.665 |
| RCA | 23 (28.4%) | 15 (29.4%) | 0.900 |
| LAD + LCX | 5 (6.2%) | 1 (2.0%) | 0.483 |
| LAD + RCA | 9 (11.1%) | 3 (5.9%) | 0.480 |
| LCX + RCA | 2 (2.5%) | 1 (2.0%) | 1.000 |
- Abbreviations: ALT, glutamic-pyruvic transaminase; AST, glutamic oxalacetic transaminase; BMI, body mass index; CAG, coronary angiography; CKD, chronic kidney disease; D-D, D-dimer; HDL-C, high density lipoprotein cholesterol; INR, international normalized ratio; LAD, left anterior descending; LCX, left circumflex; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein (a); PCI, percutaneous coronary intervention; PT, prothrombin activity; RCA, Right coronary artery; TC, total cholesterol; TG, triglyceride.
- a Data are presented as the mean ± SD, media (Q1, Q3), or n (%).
- b p < 0.05, indicating statistical significance.
- c Time refers to the duration from the onset of chest pain to the revascularization procedure.
Additionally, when CKD patients were stratified by renal function levels (eGFR), the prevalence of slow flow or no reflow after PCI were comparable across the groups (mild vs. moderate vs. severe renal dysfunction: 14.3% [4/28] vs. 35.5% [22/62] vs. 28.6% [12/42], p = 0.121).
3.3 Predictive Value of CKD and Lp(a) Levels for Coronary Slow Flow or No Reflow After PCI in STEMI Patients
Risk factors associated with coronary slow flow/no reflow after PCI in the whole STEMI cohort were identified by univariate and multivariate logistic regression models (Table 3). Elevated Lp(a) (OR = 1.725, 95% CI: 0.913–3.259, p = 0.040), LDL-C levels (OR = 1.457, 95% CI: 1.102–1.927, p = 0.008) and blood glucose levels (OR = 1.128, 95% CI: 1.001–1.257, p = 0.048) were determined to be independent risk factors for coronary slow flow/no-reflow after PCI in this STEMI cohort.
| Univariate logistic regression | Multivariate logistic regression | ||||
|---|---|---|---|---|---|
| OR | p value | OR | 95% CI | p value | |
| Elevated Lp(a) | 2.887 | 0.0001a | 1.725 | 0.913–3.259 | 0.040b |
| CKD | 2.353 | 0.002a | 1.657 | 0.880–3.122 | 0.118 |
| Male | 1.09 | 0.76 | |||
| Age (years) | 1.002 | 0.891 | |||
| BMI (kg/m2) | 0.909 | 0.025 | 0.927 | 0.848–1.014 | 0.097 |
| Smoking history | 0.682 | 0.26 | |||
| Drinking history | 0.764 | 0.496 | |||
| Diabetes | 1.857 | 0.027 | 1.228 | 0.651–2.316 | 0.526 |
| Hypertension | 1.008 | 0.979 | |||
| TC (mmol/L) | 1.384 | 0.001 | |||
| TG (mmol/L) | 1.114 | 0.288 | |||
| HDL-C (mmol/L) | 2.229 | 0.05 | |||
| LDL-C (mmol/L) | 1.510 | 0.001a | 1.457 | 1.102–1.927 | 0.008b |
| ALT (U/L) | 1.002 | 0.286 | |||
| AST (U/L) | 1.001 | 0.388 | |||
| Blood glucose (mmol/L) | 1.151 | 0.009 | 1.122 | 1.001–1.257 | 0.048b |
| Potassium (mmol/L) | 1.132 | 0.598 | |||
| Sodium (mmol/L) | 0.949 | 0.215 | |||
| INR | 3.199 | 0.311 | |||
| Fibrinogen (g/L) | 1.108 | 0.507 | |||
| PT (%) | 0.990 | 0.197 | |||
| D-D | 1.000 | 0.165 | |||
- Abbreviations: ALT, glutamic-pyruvic transaminase; AST, glutamic oxalacetic transaminase; BMI, body mass index; CI, Confidence interval; CKD, chronic kidney disease; D-D, D-dimer; HDL-C, high density lipoprotein cholesterol; INR, international normalized ratio; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein (a); OR, odds ratio; PCI, percutaneous coronary intervention; PT, prothrombin activity; STEMI, ST-elevation myocardial infarction; TC, total cholesterol; TG, triglyceride.
- a p < 0.05, indicating the variable was considered for inclusion in the multivariate model.
- b p < 0.05, indicating statistical significance in the multivariate model.
Although univariate analysis indicated CKD as a significant factor associated with coronary slow flow/no reflow after PCI (OR = 2.353, 95% CI: 1.357–4.08, p = 0.002), this association was no longer significant in the multivariate model after adjustment (p > 0.05).
3.4 Predictive Value of Lp(a) Levels for Coronary Slow Flow or No Reflow After PCI in STEMI Patients With CKD
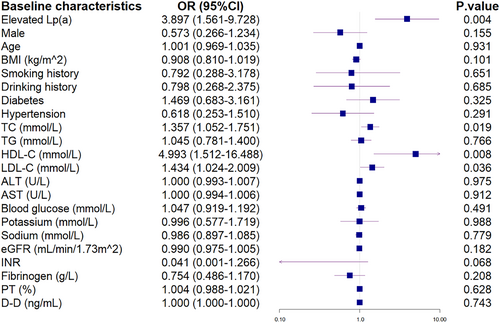
Elevated Lp(a) level (> 30 mg/dL) was observed as an independent risk factor for coronary slow flow or no reflow after PCI in STEMI patients with CKD (OR = 2.985, 95% CI: 1.134–7.853, p = 0.027; Table 4 and Figures 1 and 2). However, stratification by renal function level in these CKD patients revealed that renal function level was not a risk factor for coronary slow flow/no reflow after PCI (OR = 1.332, 95% CI: 0.782–2.268, p = 0.291).
| Univariate logistic regression | Multivariate logistic regression | ||||
|---|---|---|---|---|---|
| OR | p value | OR | 95% CI | p value | |
| Elevated Lp(a) | 3.897 | 0.004a | 2.985 | 1.134–7.853 | 0.027b |
| Male | 0.573 | 0.155 | |||
| Age | 1.001 | 0.931 | |||
| BMI (kg/m2) | 0.908 | 0.101 | |||
| Smoking history | 0.792 | 0.651 | |||
| Drinking history | 0.798 | 0.685 | |||
| Diabetes | 1.469 | 0.325 | |||
| Hypertension | 0.618 | 0.291 | |||
| TC (mmol/L) | 1.357 | 0.019a | |||
| TG (mmol/L) | 1.045 | 0.766 | |||
| HDL-C (mmol/L) | 4.993 | 0.008a | 2.916 | 0.764–11.130 | 0.117 |
| LDL-C (mmol/L) | 1.434 | 0.036a | 1.103 | 0.753–1.618 | 0.614 |
| ALT (U/L) | 1.000 | 0.975 | |||
| AST (U/L) | 1.000 | 0.912 | |||
| Blood glucose (mmol/L) | 1.047 | 0.491 | |||
| Potassium (mmol/L) | 0.996 | 0.988 | |||
| Sodium (mmol/L) | 0.986 | 0.779 | |||
| eGFR (mL/min/1.73 m2) | 0.990 | 0.182 | |||
| INR | 0.041 | 0.068 | |||
| Fibrinogen (g/L) | 0.754 | 0.208 | |||
| PT (%) | 1.004 | 0.628 | |||
| D-D (ng/mL) | 1.000 | 0.743 | |||
- Abbreviations: ALT, glutamic-pyruvic transaminase; AST, Glutamic oxalacetic transaminase; BMI, Body Mass Index; CI, Confidence interval; CKD, chronic kidney disease; D-D, D-dimer; HDL-C, High density lipoprotein cholesterol; INR, international normalized ratio; LDL-C, Low density lipoprotein cholesterol; Lp(a), Lipoprotein (a); OR, Odds Ratio; PCI, percutaneous coronary intervention; PT, Prothrombin activity; STEMI, ST-elevation myocardial infarction; TC, total cholesterol; TG, triglyceride.
- a p < 0.05, indicating the variable was considered for inclusion in the multivariate model.
- b p < 0.05, indicating statistical significance in the multivariate model.

3.5 PCSK-9 Inhibitor Therapy and Clinical Outcomes of PCI Patients With CKD and Elevated Lp(a)
Baseline clinical features of STEMI patients with CKD and elevated Lp(a) receiving PCSK-9 inhibitor therapy versus conventional therapy are summarized in Table 5. The general characteristics and biochemical parameters were comparable between the two groups. After 6 months of therapy, the reduction in Lp(a) levels was significantly greater in the PCSK-9 inhibitor therapy group compared to the conventional therapy group (median: −31.75% vs. −13.06%, p = 0.0001).
| PCSK-9 inhibitor therapy group (n = 36) | Conventional therapy group (n = 45) | p value | |
|---|---|---|---|
| General information | |||
| Male | 20 (55.6%) | 24 (53.3%) | 0.842 |
| Age (years) | 65.39 ± 10.32 | 61.53 ± 11.77 | 0.126 |
| BMI (kg/m2) | 25.17 ± 3.30 | 24.90 ± 3.33 | 0.717 |
| Smoking history | 5 (13.9%) | 9 (20.0%) | 0.470 |
| Drinking history | 4 (11.1%) | 6 (13.3%) | 1.000 |
| Diabetes | 16 (44.4%) | 26 (57.8%) | 0.233 |
| Hypertension | 27 (75.0%) | 35 (77.8%) | 0.769 |
| Assay index | |||
| TC (mmol/L) | 4.82 (3.91, 5.43) | 4.66 (4.02, 5.68) | 0.936 |
| TG (mmol/L) | 1.37 (1.03, 1.85) | 1.72 (1.42, 2.50) | 0.052 |
| HDL-C (mmol/L) | 1.17 (0.96, 1.46) | 1.15 (0.97, 1.42) | 0.812 |
| LDL-C (mmol/L) | 3.04 ± 1.11 | 3.10 ± 1.35 | 0.818 |
| Lp(a) (mg/dL) | 54.5 (37.15, 68.3) | 78.6 (46.2, 109.6) | 0.056 |
| ALT (U/L) | 17 (13.9, 26.5) | 23 (13, 29) | 0.248 |
| AST (U/L) | 19 (15, 24) | 20 (15, 29) | 0.624 |
| Blood glucose (mmol/L) | 6.74 ± 2.83 | 7.32 ± 2.75 | 0.354 |
| Potassium (mmol/L) | 4.44 ± 0.73 | 4.58 ± 0.76 | 0.377 |
| Sodium (mmol/L) | 139.54 ± 3.35 | 139.53 ± 4.83 | 0.989 |
| Creatinine (µmol/L) | 134.9 (101.35, 239.9) | 180 (143, 284.7) | 0.059 |
| eGFR (mL/min/1.73 m2) | 42.63 ± 28.12 | 32.29 ± 20.74 | 0.060 |
| INR | 1.02 ± 0.11 | 1.00 ± 0.15 | 0.585 |
| Fibrinogen (g/L) | 3.68 ± 0.86 | 3.98 ± 0.95 | 0.565 |
| PT (%) | 101.39 ± 27.63 | 103.27 ± 24.08 | 0.744 |
| D-D (ng/mL) | 440 (270, 545) | 360 (300, 540) | 0.594 |
| Use of beta-blockers | 23 (63.9%) | 33 (73.3%) | 0.361 |
| Use of ezetimibe | 23 (63.9%) | 26 (57.8%) | 0.576 |
| Use of calcium channel blockers | 27 (75.0%) | 33 (73.3%) | 0.865 |
| Post-therapy | |||
| TC (mmol/L) | 3.57 (3.19, 4.16) | 3.85 (3.52, 4.35) | 0.102 |
| TG (mmol/L) | 1.39 (1.19, 1.87) | 1.52 (1.15, 1.87) | 0.546 |
| HDL-C (mmol/L) | 1.25 (0.98, 1.52) | 1.14 (0.98, 1.24) | 0.177 |
| LDL-C (mmol/L) | 2.02 ± 0.73 | 2.26 ± 0.52 | 0.102 |
| Lp(a) (mg/L) | 33.95 (24.60, 54.80) | 75.10 (32.50, 92.50) | 0.002b |
| eGFR (mL/min/1.73 m2) | 42.10 ± 21.22 | 36.41 ± 30.40 | 0.334 |
| Curative effect | |||
| TC (mmol/L) | −21.32 ± 24.90 | −17.50 ± 21.39 | 0.46 |
| TG (mmol/L) | −1.23 ± 34.09 | −12.88 ± 26.75 | 0.089 |
| HDL-C (mmol/L) | 4.92 ± 20.41 | −4.07 ± 19.59 | 0.047b |
| LDL-C (mmol/L) | −29.98 ± 21.77 | −21.63 ± 20.29 | 0.079 |
| Lp(a) (mg/dL) | −31.75 ± 17.54 | −13.06 ± 17.73 | 0.0001b |
| MACCEs | |||
| Three months | 1 (2.8%) | 4 (8.9%) | 0.502 |
| Six months | 4 (11.1%) | 8 (17.8%) | 0.401 |
| Twelve months | 8 (22.2%) | 23 (51.1%) | 0.008b |
| Readmission for Angina | 2 (5.6%) | 11 (24.4%) | 0.031b |
| Readmission for heart failure | 3 (8.3%) | 4 (8.9%) | 1.000 |
| Nonfatal myocardial infarction | 0 (0%) | 5 (11.1%) | 0.110 |
| Nonfatal stroke | 0 (0%) | 2 (4.4%) | 0.575 |
| Cardiac death | 1 (2.8%) | 0 (0%) | 0.910 |
| Revascularization | 2 (5.6%) | 1 (2.2%) | 0.844 |
| Renal function deterioration | |||
| Three months | 0 (0%) | 0 (0%) | — |
| Six months | 1 (2.8%) | 3 (6.7%) | 0.774 |
| Twelve months | 2 (5.6%) | 6 (13.3%) | 0.429 |
| Hemorrhage | |||
| Three months | 2 (5.6%) | 0 (0%) | 0.379 |
| Six months | 3 (8.3%) | 0 (0%) | 0.167 |
| Twelve months | 3 (8.3%) | 0 (0%) | 0.167 |
- Abbreviations: ALT, glutamic-pyruvic transaminase; AST, glutamic oxalacetic transaminase; BMI, body mass index; CKD, chronic kidney disease; D-D, D-dimer; eGFR, estimate glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; INR, international normalized ratio; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein (a); MACCEs, major adverse cardio-cerebrovascular events; PCSK-9, proprotein convertase subtilisin/kexin type 9; PT, prothrombin activity; TC, total cholesterol; TG, triglyceride.
- a Data are presented as the mean ± SD, median (Q1, Q3), or n (%).
- b p < 0.05, indicating statistical significance.
During 1-year follow-up, the prevalence of MACCEs was significantly lower in the PCSK-9 inhibitors therapy group than in the conventional therapy group (22.2% vs. 51.1%, p = 0.008). Readmission due to angina pectoris was also markedly reduced in the PCSK-9 inhibitors therapy group compared to the conventional therapy groups (5.6% vs. 24.4%, p = 0.031).
In addition, adverse events such as worsening renal function occurred in 5.6% (n = 2) of patients and bleeding in 8.3% (n = 3) in the PCSK-9 inhibitor therapy group, compared to 13.3% (n = 6) and 0% (n = 0), respectively, in the conventional therapy group (all p > 0.05).
At the 12-month follow-up, re-angiography was performed in 27 patients with post-PCI slow flow or no reflow (1 patient died of myocardial infarction, and 3 refused re-angiography), revealed that coronary slow flow was improved in patients receiving PCSK-9 inhibitors compared to those on conventional therapy [57.1% (8/14) vs. 15.4% (2/13), p = 0.025].
4 Discussion
The main finding of this study was that Lp(a) levels were significantly increased in STEMI patients with CKD compared with patients with non-CKD. Notably, in STEMI patients with CKD, elevated Lp(a) was identified as an independent risk factor for coronary slow flow or no reflow after PCI. Additionally, PCSK-9 inhibitors were shown to effectively reduce Lp(a) levels and improve coronary slow flow in STEMI patients with elevated Lp(a).
4.1 Lp(a) With CKD
Lp(a) levels are primarily determined by genetic factors, but kidney function is one of the few nongenetic factors known to influence plasma Lp(a) levels. Elevated Lp(a) is a hallmark of dyslipidemia specific to renal insufficiency [4]. This effect is particularly pronounced with high molecular weight apolipoproteins (a), providing strong evidence for the role of kidney in Lp(a) catabolism [21]. Renal insufficiency in patients with CKD contributes to elevated Lp(a) levels through a dual mechanism of reduced clearance and increased synthesis, independent of genetic factors [22, 23]. Consistent with previous findings, our results demonstrated significantly higher Lp(a) levels in CKD patients compared to non-CKD patients (median 36.75 vs. 15.90 mg/dL, p = 0.0001). Furthermore, Lp(a) levels increased progressively with worsening renal function (median 24.60 vs. 32.60 vs. 55.45 mg/dL, p = 0.002).
On the other hand, previous studies have shown that high Lp(a) levels are not only a consequence but also a contributing factor to CKD [4]. Lp(a) can promote kidney damage through several mechanisms: (1) Lp(a) contains low-density lipoprotein particles that oxidize upon entering the vascular wall, becoming highly immunogenic and pro-inflammatory oxidized LDL, which can damage renal tissue. (2) The apolipoprotein (a) component of Lp(a), can induce microvascular injury, further impairing renal function [21, 24]. (3) Dysregulation of Lp(a) metabolism can damage the glomeruli and tubulointerstitial structures of the kidney [25]. In summary, the interplay between elevated Lp(a) and CKD exacerbates renal dysfunction and amplifies the risk of cardiovascular events [4].
4.2 CKD and Slow Flow/No Reflow After PCI
Previous studies have shown a significant association between reduced renal function and impaired coronary blood flow reserve [12, 15], with CKD identified as an independent risk factor for coronary slow flow/no reflow [26]. This relationship has been confirmed across varying grades of CKD [15, 27, 28]. Adam et al. demonstrated a marked reduction in coronary flow velocity reserve in patients with chronic renal insufficiency compared to those with normal renal function (2.34 ± 0.4 vs. 3.05 ± 0.3, p = 0.003) [27]. Similarly, Akin and colleagues reported that eGFR was significantly correlated with slow coronary blood flow (OR = 0.972, 95% CI: 0.957–0.987, p < 0.001) [15].
Consistent with prior studies, our findings revealed that slow or no reflow after PCI was significantly more common in the CKD group than in the non-CKD group (28.79% vs. 14.66%, p = 0.002). However, multivariate regression analysis indicated that different grades of renal dysfunction were not independent predictors of slow flow/no reflow after PCI in CKD patients (OR = 1.997, 95% CI: 0.865–4.611, p = 0.105). Prior studies have implicated factors such as preoperative blood glucose, total cholesterol, D-Dimer, and fibrinogen levels no reflow after PCI [29]. In our study, multivariate regression analysis confirmed that elevated Lp(a) and blood glucose remained associated with slow flow of no reflow after PCI in our STEMI cohort. Interestingly, despite disrupted lipid metabolism, LDL-C levels were comparable between the two groups (2.76 ± 1.15 vs. 2.68 ± 1.00 mmol/l, p = 0.489). This finding may be attributed to the complex interplay between lipid metabolism disorders and the inflammatory response in patients with CKD.
4.3 Lp(a) and Slow Flow/No Reflow After PCI
The 2022 EAS Consensus Statement proposes that Lp(a) concentrations > 50 mg/dL are associated with clinically significant increase in cardiovascular risk, with a “grey zone” between 30 and 50 mg/dL [30]. While the 2023 Chinese Lipid Management Guidelines identify Lp(a) levels > 30 mg/dL as increasing the risk of arteriosclerotic coronary artery disease [31]. Moreover, elevated Lp(a) levels have been linked not only to the severity of coronary arterial stenosis, but also to worse clinical outcomes after PCI [32]. In our study, elevated Lp(a) was identified as an independent risk factor for slow flow or no reflow after PCI in patients with chronic renal insufficiency (OR = 2.985, CI: 1.134–7.853, p = 0.027). This aligns with previous evidence suggesting that Lp(a) contributes to endothelial injury [33] and thrombosis [34, 35], mechanisms that could underlie slow flow or no reflow.
Our data demonstrated that patients with elevated Lp(a) levels exhibited enhanced clotting function, as evidenced by higher fibrinogen levels (3.93 ± 0.91 vs. 3.43 ± 0.81 g/L, p = 0.002), increased prothrombin activity (102.42 ± 25.57% vs. 93.78 ± 16.41%, p = 0.019), and shorter clotting times (INR: 1.01 ± 0.13 vs. 1.05 ± 0.11, p = 0.038). We hypothesize that, in CKD patients, the interplay between renal dysfunction and lipid metabolism disorders exacerbates Lp(a) elevation, contributing to both inflammatory responses and endothelial damage. These mechanisms likely facilitate coronary slow flow/no reflow. Additionally, Lp(a) may promote coagulation and thrombosis, further compounding the risk of these events in STEMI patients with CKD following PCI.
4.4 PCSK-9 Inhibitors and Outcomes
PCSK-9 inhibitors effectively reduce residual lipid levels, stabilize atherosclerotic plaques, and improve coronary blood flow and myocardial perfusion [36]. These benefits are supported by clinical trials that demonstrated the efficacy of PCSK-9 inhibitors in significantly reducing LDL-C levels an improving cardiovascular outcomes [37-39]. In our study, PCSK-9 inhibitors were associated with a significant reduction in Lp(a) levels in CKD patients with elevated Lp(a) (−31.75% vs. −13.06%, p = 0.0001) and a lower prevalence of MACCEs over 1 year (22.2% vs. 51.1%, p = 0.008), particularly angina pectoris (8.3% vs. 24.4%, p = 0.031). However, it remains unclear whether these benefits are primarily attributable to Lp(a) reduction or the greater LDL-C reduction.
4.5 PCSK-9 Inhibitors and Slow Flow/No Reflow After PCI
A study by Ji and colleagues involving 120 patients with acute coronary syndrome (ACS) demonstrated that PCSK-9 inhibitors combined with statins significantly improved microcirculation function and coronary blood flow after PCI. At 6 months after PCI, the PCSK-9 inhibitor group had a significantly higher proportion of patients achieving grade 3 coronary flow compared to the control group at 6 months after PCI (98.2% vs. 89.1%, p = 0.04) [40]. Similarly, in our study, at the 12-month follow-up, re-angiography was performed in 27 patients with post-PCI slow flow or no reflow, revealing that revealing improved coronary blood flow in the PCSK-9 inhibitor group compared to the conventional therapy group [57.1% (8/14) vs. 15.4% (2/13), p = 0.025]. However, the primary outcome of coronary blood flow improvement was based on a limited sample of patients undergoing repeat angiography, underscoring the need for further studies with larger cohorts.
Although the mechanisms by which PCSK-9 inhibitors improve coronary blood flow are not fully elucidated, potential associations may include: PCSK-9 inhibitors reduce LDL-C and other residual lipids [36, 41]. (2) Reduced platelet reactivity: Cristina Barale and colleagues observed a reduction in platelet reactivity during a 1-year follow-up of PCSK-9 inhibitor therapy [42]. (3) Enhanced microcirculation function: Combined with statins, PCSK-9 inhibitors improve microvascular function in ACS patients post-PCI [40]. (4) Anti-inflammatory effects and improved endothelial function: PCSK-9 inhibitors mitigate inflammation and enhance endothelial cell function, further supporting coronary blood flow [43]. Further studies are warranted to clarify the specific mechanisms by which PCSK-9 inhibitors improve coronary blood flow, particularly in STEMI patients with CKD.
4.6 Study Limitations
This study has several limitations. First, as a retrospective, observational analysis, it is inherently subject to biases due to unmeasured confounding variables that may affect the results. Second, the single-center design and relatively small sample size limit the generalizability of our findings to broader patient populations. Additionally, the primary outcome of coronary blood flow improvement was based on a small subset of patients who underwent repeat angiography, which underscores the need for larger studies to confirm these results. Moreover, it remains unclear whether the observed clinical benefits are primarily due to Lp(a) reduction or the more significant LDL-C reduction achieved with PCSK-9 inhibitors, necessitating further investigation. To address these limitations, future research should include multicenter studies with larger sample sizes and randomized controlled trials to establish causal relationships and evaluate the long-term effects of Lp(a) reduction and PCSK-9 inhibitor therapy in this patient population.
5 Conclusions
Elevated Lp(a) levels are independent predictors of slow flow or no reflow after PCI in STEMI patients with CKD. PCSK-9 inhibitors effectively reduce Lp(a) levels, and preliminary evidence suggests they may improve coronary blood flow and reduce cardiovascular events. However, due to the small sample size and nonrandomized design, further studies are needed to confirm these findings. Monitoring Lp(a) levels and considering early PCSK-9 inhibitor therapy in patients with CKD may help prevent slow flow or no reflow after PCI.
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions. This work was supported by Natural Science Foundation of Shandong Province (ZR2023MH083) and Shandong Traditional Chinese Medicine Science and Technology Project (NO.2022078).
Ethics Statement
The ethical approval was obtained from the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 29131). Informed consent was obtained from all patients. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.




