Enhanced Efficiency of Sequential Cutting Balloon Angioplasty in Calcified Coronary Artery Disease: The RODIN-CUT Technique
ABSTRACT
Background
Heavily calcified coronary artery disease presents significant challenges in percutaneous coronary intervention (PCI), often requiring advanced techniques to achieve optimal outcomes. Cutting balloons (CB) have shown potential for plaque modification; however, their effectiveness is limited without standardized protocols.
Aims
This study introduces the RODIN-CUT technique, a novel approach utilizing sequential CB inflations with real-time intravascular ultrasound (IVUS) guidance. The technique aims to enhance calcified plaque modification, improve stent expansion, and achieve consistent procedural success in heavily calcified coronary lesions.
Methodology
A retrospective analysis of three consecutive patients requiring specialized approaches beyond conventional PCI was included at three centers in Belgium. The RODIN-CUT protocol involved multiple CB inflations at precise lesion segments, followed by immediate IVUS imaging after each inflation to assess plaque modification and guide further therapy. Procedural success was defined as residual stenosis < 30% with TIMI 3 distal flow. The study evaluated the dose-dependent effects of repeated CB inflations on plaque fracture depth and distribution.
Conclusion
The RODIN-CUT technique demonstrated promising outcomes, achieving procedural success in all cases with enhanced stent expansion and minimal complications. The technique's simplicity, cost-effectiveness, and reproducibility make it a viable option for treating heavily calcified coronary lesions. Further large-scale studies are required to validate these findings and establish the RODIN-CUT technique as a standard approach for complex calcified lesions.
1 Introduction
Moderate to severe calcifications are found in about one-third of the coronary lesions in patients with stable ischemic heart disease and acute coronary syndromes [1, 2]. Coronary artery calcifications (CACs) are markers of poor prognosis in coronary artery disease [3], posing challenges for percutaneous coronary interventions (PCI) by limiting stent expansion and increasing stent failure risk [4-7].
Effective lesion preparation and breaking calcifications are essential to increase vessel compliance and ensure proper stent expansion [8]. However, the optimal angioplasty technique for different morphologies of the calcified lesions remains unclear [9]. The CB, equipped with microsurgical blades (atherotomes), is designed to make endovascular incisions and fracture calcium during inflation [10-13]. The Wolverine CB (Boston Scientific) has shown better lumen gain, larger final minimal stent area (MSA), and more symmetric stent expansion than conventional balloon angioplasty in calcified lesions [14-17].
Endovascular imaging studies highlight the necessity of fracturing calcified plaque to prevent incomplete stent expansion and suboptimal outcomes [18]. Lesions with calcium fractures show significantly larger stent expansion than those without [19]. The CB's ability to fracture calcified lesions is crucial, yet clinical studies are limited due to difficulties in precisely measuring the degree of plaque modification after each dilatation [20].
The arc of calcium, length, and thickness of the calcification, as well as technical aspects like inflation pressure, blade position, and the number of inflations, influence CB predilatation effectiveness.
This report introduces the RODIN-CUT technique, which uses a CB in a specific manner for treating heavily calcified coronary disease, illustrated in four calcified coronary lesions. To fully understand the effect of this approach at the lesion level, a simple methodology involving immediate intravascular ultrasound (IVUS) evaluation after each CB inflation was applied, emphasizing multiple inflations and precise positioning of the blades. The name of this technique pays tribute to the meticulous and careful work of the famous sculptor Auguste Rodin (1840–1917), where repetition of exact movements is key when it comes to manipulating hard materials.
2 The RODIN-CUT Protocol
The RODIN CUT is a systematic approach designed to assess the effects of the CB on calcified plaques, using IVUS (Opticross HD 60 MHz, Boston Scientific, USA) to capture detailed imaging after each inflation. The procedure needs a 7 Fr guiding catheter (GC) which can accommodate both the CB and the IVUS probe (Central Illustration 1). The target distal vessel is double-wired using two workhorse guidewires for the simultaneous use of both devices.
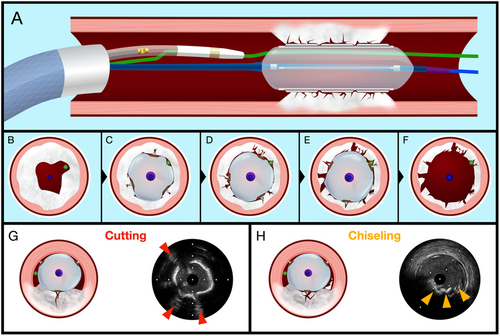
An initial IVUS assessment is performed after introducing the IVUS catheter into the GC and advancing it to the distal vessel, where a baseline pullback is conducted to capture initial images of the lesion. After this baseline imaging, the IVUS probe is completely pulled back from the GC and the CB is positioned at the lesion site. The next step is the reinsertion of the IVUS catheter inside the GC up to its distal tip.
The main part of the technique involves inflating the CB at the lesion with pressures ranging between 16 and 18 atm for 5–10 s per inflation. After each inflation, the CB is advanced distally, allowing the IVUS catheter to cross the investigation site and capture images of the plaque's response. Following each imaging sequence, the IVUS probe is retracted to a safe position—either in the proximal-ostial part of the vessel or back inside the GC—to prevent damage from the CB's atherotomes. This approach allows for a detailed evaluation of changes in the plaque after each inflation.
The cycle of CB inflation, advancement, and subsequent IVUS imaging is repeated at least five times to ensure a comprehensive assessment. After each inflation, the CB is repositioned, and new IVUS images are obtained, offering a progressive view of how the plaque structure changes with each application of pressure.
Before employing the CB, standard angioplasty with a noncompliant (NC) balloon is performed, to facilitate the device crossing.
The extensive imaging data collected after each inflation cycle allows for an in-depth analysis of the plaque's evolution, including the creation of fissures, the length of fractures, and the dimensions of entry points.
3 Methods
3.1 Data Collection and Patient Selection
We conducted a retrospective analysis of three consecutive patients who underwent PCI for the treatment of highly calcified coronary artery disease at Jolimont Hospital in La Louvière, Belgium, Ziekenhuis Aan de Stroom (ZAS) Middelheim in Antwerp, Belgium, and Arlon Hospital, Belgium. The selection criteria included patients with severe calcification, identified as requiring specialized techniques beyond conventional PCI due to the extent of calcified plaques. All patients were treated using the Rodin-CUT technique, a method designed for enhanced lesion modification and improved stent expansion in challenging calcified vessels.
The primary goal of the intervention was to achieve procedural success, defined as reducing residual stenosis to less than 30% and ensuring a distal TIMI 3 flow.
3.2 Case Descriptions
3.2.1 Lesion 1
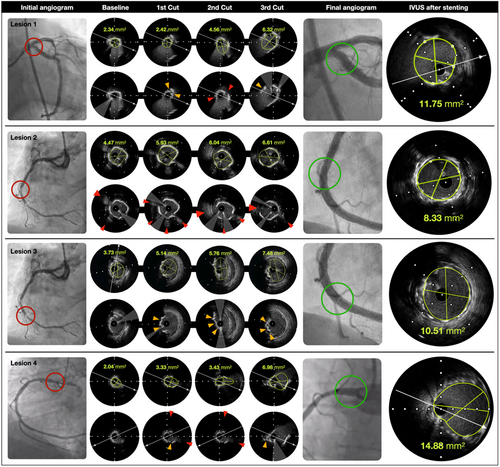
An 84-year-old male patient with a medical history of hypertension, diabetes mellitus, and dyslipidemia underwent PCI for a severely calcified ostial lesion of the left circumflex coronary artery (LCX) (Figure 1). The lesion presented a minimal lumen area (MLA) of 2.34 mm² and a length of 2.5 mm, with an arc of calcium of 340° (Figure 2).
A dedicated protocol is outlined in detail in the technical description of the Rodin-CUT, where the procedural steps, device setup, and imaging sequence are comprehensively described.
The results from the IVUS evaluation showed a progressive increase of the MLA with each inflation: 2.34 - 2.42 - 4.56 - 6.32 mm² (Figure 1. Lesion 1. Baseline; first to third Cut). The arc of calcium decreased gradually from 340° to 180°, 160°, and finally 120°. The number of fissures increased from 0 to 1, 3, and 5, respectively. The entry dimensions of the fissures also increased: 0.4 - 0.76 - 0.98 mm (Figure 1. Lesion 1. Baseline; first to third Cut). An important aspect was the presence of complete separation of the plaque into multiple smaller fragments. No major dissections were noticed. The angiogram and IVUS run demonstrated substantial expansion of the lesion with a mean stent area (MSA) of 11.75 mm² (Figure 1. IVUS after stenting) and excellent coronary flow. The operator preferred treatment with a drug-eluting balloon (DCB) to avoid a complex left main PCI and the subsequent risk of target lesion failure (TLF) at the level of the LCX ostium. The clinical follow-up at 6 months was satisfactory, with a significant reduction in angina symptoms.
3.2.2 Lesions 2
A 70-year-old male patient with a medical history of hypertension and dyslipidemia underwent PCI for two severe calcified lesions in the right coronary artery (RCA) (Figure 1. Lesion 2 and 3), after ischemia on stress echocardiography. A 7Fr GC was used similarly as in the previous case, following the technical steps outlined for the RODIN-CUT. The initial pullback of IVUS, revealed two different calcified lesions: one more proximal with an almost concentric ring of calcium of 340°, an MLA of 4.47 mm², and a length of 2.5 mm (Figure 1. Lesion 2. Baseline). In the presence of severe calcifications, the CB (3 × 15 mm) was used upfront.
For the circumferential calcified lesion, the MLA increased with each inflation: 4.47, 5.91, 5.56, 6.04, 6.61 mm² (Figure 1. Lesion 2. Baseline; first to third Cut). The calcium arc decreased gradually from 340° to 200°, 160°, and finally 120°. The number of fissures increased from 0 to 2, 3, and 4, respectively. The entry dimensions of the fissures also increased: 0.5, 0.66, 1.18 mm. The same aspect of complete fragmentation of the plaque into multiple smaller fragments was noticed as in the previous case (Figure 1. Lesion 2. Baseline; first to third Cut).
3.2.3 Lesion 3
The same patient with lesion 2 had a second more distal lesion about 20 mm beyond the proximal one with a nodule-like morphology and an MLA of 3.73 mm² (Figure 1. Lesion 3. Baseline). While treating this distal nodular lesion following the same steps, the MLA also increased: 3.73, 5.14, 5.76, 7.48 mm² (Figure 1. Lesion 3. Baseline; first to third Cut). No major dissections were noticed. The angiogram and IVUS run demonstrated substantial expansion of the lesion after successfully placing a DES with an MSA of 10.51 mm² (Figure 1. Lesion 3. IVUS after stenting) at the level of the nodule and an eccentricity index of 0.85.
Interestingly, in addition to the deep cracks in thinner calcium, calcified nodules experienced more superficial fragments.
3.2.4 Lesion 4
A 76-year-old male patient with a history of DES implantation in the LAD and recurrent angina 2 years postintervention was scheduled for a PCI to address a severely calcified lesion in the ostial RCA (Figure 8). A 7Fr GC was utilized similarly to the previous case, adhering to the procedural steps of the RODIN-CUT as previously described.
The ostial RCA lesion was short, with an MLA of 2.04 mm², a length of 3 mm, and an arc of calcium of 310° (Figure 1. Lesion 4).
A CB (3.5 × 15 mm) was used initially. After multiple inflations at high pressure (16–18 atm), the MLA increased progressively from 2.04 to 3.33, 3.43, and finally 6.98 mm² (Figure 1. Lesion 4. Baseline; first to third Cut). The calcium arc decreased from 310° to 200°, 180°, and eventually 120°. The number of fissures increased from 0 to 3, 4, and 5, respectively. The entry dimensions of the fissures also increased from 0.4 to 0.76 to 0.98 mm (Figure 1. Lesion 4. Baseline; first to third Cut). As in previous cases, a complete separation of the initial plaque into multiple smaller fragments was observed. No major dissections were noted. The final angiogram and IVUS run demonstrated optimal stent expansion, with an MSA of 14.88 mm² and an eccentricity index of 0.86 (Figure 1. Lesion 4. IVUS after stenting).
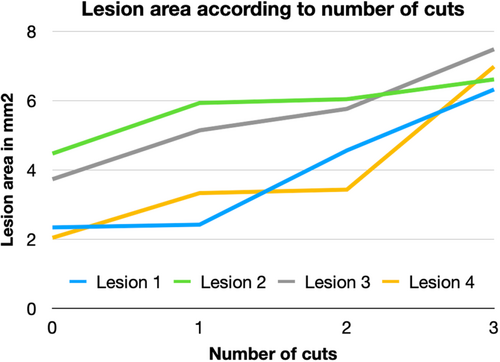
The data presented in Table 1, including all four lesions, reveal a progressive and consistent increase in the Minimum Lumen Area (MLA) with each cutting balloon (CB) inflation, as observed through intravascular imaging. For each lesion, area measurements were taken at various time points following successive inflations, demonstrating a clear, incremental expansion of the lumen. The first inflation produced a small, superficial incision with limited area expansion. However, subsequent inflations had a cumulative effect, significantly enlarging the cross-sectional area as deeper fissures and more substantial disruptions developed within the calcified plaque.
| Number of cuts | 0 | 1 | 2 | 3 | MSA | Expansion (%) | Reference segment diameter (mm) | Cutting balloon size/length (mm) |
|---|---|---|---|---|---|---|---|---|
| Lesion 1 | 2.34 | 2.42 | 4.56 | 6.32 | 11.75 | 502 | 3.24 | 3/15 |
| Lesion 2 | 4.47 | 5.93 | 6.04 | 6.61 | 8.33 | 186 | 3.17 | 3/15 |
| Lesion 3 | 3.73 | 5.14 | 5.76 | 7.48 | 10.51 | 282 | 3.63 | 3/15 |
| Lesion 4 | 2.04 | 3.33 | 3.43 | 6.98 | 14.88 | 729 | 3.25 | 3/15 |
| Mean | 3.15 | 4.21 | 4.95 | 6.85 | 11.37 | 425 | N/A | N/A |
| STDEV | 1.15 | 1.61 | 1.20 | 0.50 | 2.74 | 242 | N/A | N/A |
- Abbreviation: MSA, minimal surface area.
4 Discussion
This report introduces the RODIN-CUT technique, which implies the use of a CB in a specific way for heavily calcified coronary disease. To fully understand the effect of this new approach at the lesion level, an original and simple methodology was applied using immediate evaluation after every single inflation of the CB (Central Illustration 1). The RODIN-CUT combines three novel key technical aspects to apply mechanical stress to calcified coronary lesions using the blades of a CB. The main difference to previous CB use reports is that the RODIN-CUT requires multiple inflations, in some cases up to 15, with a minimum of five inflations depending on the effect seen in IVUS. The position of the atherotomes and the inflation pressure are further important for the optimal effect of the device. Alternating the CB and the IVUS in the same segment of the vessel causes partial rotation of the CB and its blades and a different position in each inflation. This allows for additional and different cuts in the calcified part of the lesion. Finally, the use of higher pressures than recommended by the IFU (up to 20 atm) seems safe to apply and results in higher effectivity in thicker lesions.
We named this technique in honor of the famous sculptor Auguste Rodin, whose work required meticulous and delicate maneuvers to transform hard materials like rock into beautiful, detailed masterpieces. Similarly, this technique involves multiple precise and careful steps to effectively treat heavily calcified coronary lesions. Building on this concept, a single inflation of a CB may not be sufficient for optimal vessel preparation before stent implantation.
The CB's efficacy in facilitating calcium cracking is primarily due to these protrusive elements (atherotomes), which exert focused stress on the lesion of approximately 160.7 MPa. This is significantly higher than the 65.4 MPa generated by conventional balloons without blades [21].
The table (Figure 2) highlights the dose-dependent response of the lesions to repeated CB inflations. The progressive increase in luminal surface area observed across all lesions suggests that a single inflation may not be sufficient to fully modify dense calcified structures. Instead, multiple inflations enable multiple cracks and more effective fragmentation of the plaque, leading to a stepwise enlargement of the luminal area. This is likely due to the continuous mechanical stress induced by each inflation, which progressively weakens the structural integrity of the calcified plaque, also known as the creep effect [22, 23]. The expansion was not linear but showed irregular improvement with each inflation, suggesting that the cumulative mechanical force applied by the CB allows for more effective lesion preparation.
The most relevant finding remains the increasing number of fractures, deepening cracks within the lesions, larger intraluminal entry points of the fractures, and, in some cases (specifically lesions 3 and 4), full-thickness fractures reaching the base of the calcifications. These changes were accompanied by significant increases of the MLA. Even in circular, heavily calcified lesions, as observed for lesions 1, 2, and 4, the MLA increased considerably. (Figure 1).
The current recommendations for the use of CB suggest inflations at nominal pressures of 6 atm (RBP 12 atm). However, this strategy may limit the efficacy of the device. Inflating the CB at higher pressures has been shown to enhance its effectiveness in treating calcified lesions [21]. This was elegantly demonstrated in the COPS randomized trial [24], where high-pressure CB inflation was safe and was associated with a larger MSA and lower stent eccentricity at the calcium site compared to noncompliant balloons (NCB).
The high-pressure CB strategy was also employed in the PREPARE-CALC-COMBO study [25]. This study showed that using rotational atherectomy (RA) in combination with high-pressure CB inflation resulted in higher acute lumen gain and a larger MSA compared to using the Rotablator (Boston Scientific) alone. These findings highlight the benefit of high-pressure CB inflation in optimizing procedural outcomes in heavily calcified coronary lesions.
An important observation was the absence of major complications, such as coronary perforation or flow-limiting dissections, despite multiple high-pressure inflations. The CB creates controlled incisions, which may prevent major uncontrolled dissections typically associated with angioplasty [26]. Proper sizing of the CB is crucial to avoid complications. The primary task of the CB is to fracture the calcified plaque without exerting excessive force on other structures of the coronary wall [27]. Therefore, maintaining an appropriate balloon-to-lumen (BL) ratio at the site of the MLA and maximum of the calcifications is essential. Downsizing the CB by 0.5 mm compared to the media-to-media diameter at the level of maximum calcifications on IVUS is advisable to prevent vessel rupture.
The two main morphological features of calcium that independently contribute to PCI failure are nodular calcifications and circular lesions, both of which hinder proper dilatation [28-33]. Despite the availability of various calcium modification devices—such as orbital and rotational atherectomy, and intravascular lithotripsy—stent expansion and symmetry, associated with worse long-term clinical outcomes, remain challenging to achieve in these types of calcified lesions [5, 34, 35]. Thus, the optimal and targeted use of each debulking device in the most efficient manner is crucial for achieving the best possible PCI results.
Our hypothesis on how calcified lesions respond to repetitive use of the CB is based on a chiseling effect. The CB's atherotomes apply concentrated mechanical stress, scoring and fracturing the calcified plaque by creating micro-cracks. These fissures spread through weaker points, where the highest stress levels are achieved within the thinnest part of the calcification, similar to a chisel breaking a rock, resulting in surface erosion and thinning of the plaque rather than deep cracks.
In nodular calcifications, the CB's blades focus tensile stress on the raised, rigid regions, causing fractures that extend radially. The chiseling process causes multiple controlled brittle fractures, which contrasts with rotational atherectomy, which grinds the surface continuously. Multiple inflations eventually result in an effective breakdown of nodular calcified plaques.
For circular calcifications, the CB creates focused radial stress with controlled breaking of the ring of calcium increasing the vessel compliance. The fragments can then be shifted by balloon dilatation, similar to the movement of tectonic plates.
Multiple inflations may be required, as repetition is necessary to overcome high resistance and create deep fractures.
In current practice, there is a lack of consensus and evidence from intravascular imaging, on whether a calcified lesion has been adequately prepared for stent implantation. To address this gap, we have introduced a parameter termed “Plaque Decimation Strategy (PDS)” emphasizing the continuous fragmentation of atherosclerotic plaque for maximal vessel compliance, and optimal stent expansion.
To achieve optimal stent expansion, it is crucial to understand that merely creating a single superficial fissure in a calcified coronary plaque is often insufficient. Instead, there must be a complete and thorough separation of the plaque into multiple smaller fragments. The mechanism of plaque preparation with the RODIN-CUT technique resembles the mechanism of action of rotary drill bits used for rock drilling, where a rolling cone with small chisels is used to crack hard rock.
The CB offers several technical advantages, including ease of use and minimal need for specific training. It is readily available and cost-effective compared to other devices designed for calcium modification. The major limitation of the CB could be its profile. However, the newer generation CB (Wolverine) features a smaller crossing profile than its predecessor and has shown a high delivery success rate in clinical practice, comparable to scoring balloons [36]. A major limitation is the unpredictable presence and size of shadowing created by the two guidewires, which makes it difficult to accurately count and assess the fissures.
5 Conclusion
The novel RODIN-CUT technique aims to promote a standardized use of CB to enhance their efficiency in treating calcified coronary artery disease. According to our initial clinical experience, a dose-dependent effect in regard to multiple inflations was seen for CBs using this new method. The immediate availability of IVUS allows targeted therapy with multiple inflations of a CB at various positions of the lesion and provides valuable insights into the mechanistic preparation of calcified lesions.
Further large-scale clinical trials are necessary to precisely determine the role and benefits of this approach in the management of calcified coronary lesions.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




