Closure of a Late-Onset Iatrogenic Left Ventricular Pseudoaneurysm Caused by Erosion of an Apical Muscular VSD Device
ABSTRACT
We report the case of a 3-year-old asymptomatic girl (12 kg, 96 cm) who was diagnosed with a large iatrogenic left ventricular pseudoaneurysm (LVP) on follow-up ultrasound, 14 months after apical muscular ventricular septal defect (VSD) closure with a 10 mm Amplatzer Muscular VSD occluder (Abbott, USA) due to device erosion. The LVP was successfully occluded using detachable Penumbra coils, with complete thrombo-exclusion confirmed at 12-month follow-up.
1 Introduction
Pediatric left ventricular outpouchings (LVOs), whether congenital or acquired, are rare anomalies that may occur in isolation or alongside cardiovascular malformations and are classified as diverticula, aneurysms, or pseudoaneurysms [1-3]. Unlike adults, where myocardial infarction is the primary cause, trauma—particularly from motor vehicle accidents—is the leading cause of LVOs in children [1]. The incidence of iatrogenic device-related left ventricular pseudoaneurysms (LVP) is unknown due to their rarity and underdiagnosis. Pseudoaneurysms, sometimes linked to hardware manipulation after perventricular ventricular septal defect (VSD) closure [4, 5], can develop when cardiac erosion is confined by the adherent pericardium, posing a risk of rupture and sudden cardiac death if untreated [6, 7]. Surgical resection and percutaneous closure are both considered viable therapeutic options, depending on the specifics of each case [4-12]. We present the case of a 3-year-old girl who developed an iatrogenic LVP due to erosion of an apical muscular VSD device 14 months postprocedure, successfully occluded with coil implantation.
2 Case Presentation
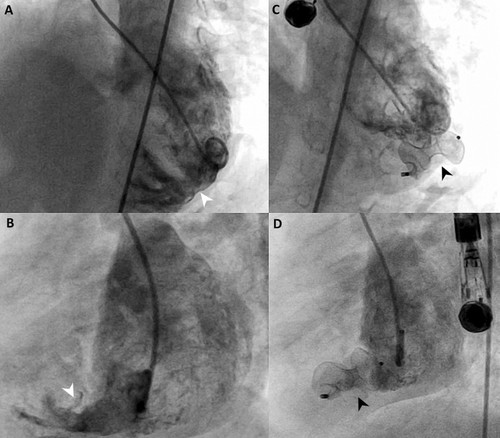
A 3-year-old asymptomatic girl (12 kg, 96 cm) with a history of large inlet and apical muscular VSDs and a hypoplastic aortic arch with coarctation underwent surgical arch repair and pulmonary artery banding at 1 week old. At 1 year of age, her 8 mm large apical VSD, which was challenging for surgical repair, was successfully closed percutaneously using a 10 mm Amplatzer Muscular VSD Occluder device (Abbott, USA) without complications (Figure 1, Supporting Information S1: Video 1). Following this, the pulmonary artery band was removed, and the inlet VSD was surgically closed. She exhibited normal growth, no symptoms, and serial echocardiograms confirmed a well-seated device, no residual leak, and normal left ventricle (LV) function and dimensions.
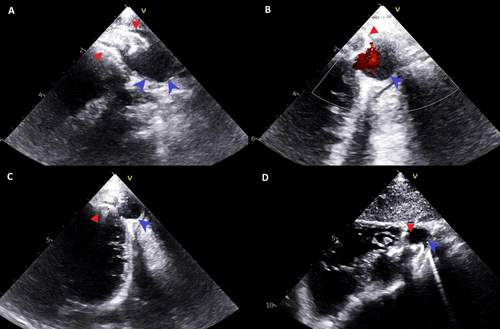
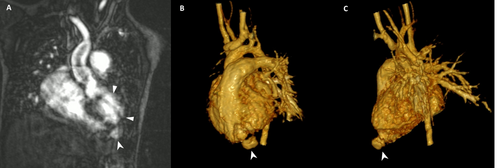
Fourteen months postsurgery, routine echocardiography revealed a 15 × 15 mm anterior-apical LVO with a thin, dyskinetic wall and passive filling during systole (Figure 2, Supporting Information S2: Video 2). Concerns arose about device erosion, as part of the left retention disk appeared to sit within the LVO neck. Despite this, the child remained asymptomatic with good LV function, no pericardial effusion, and stable LVO size over 8 months of follow-up. A cardiac MRI performed for anatomical, functional, and tissue evaluation was limited by device-related artifacts, suggesting an LVP (Figure 3).
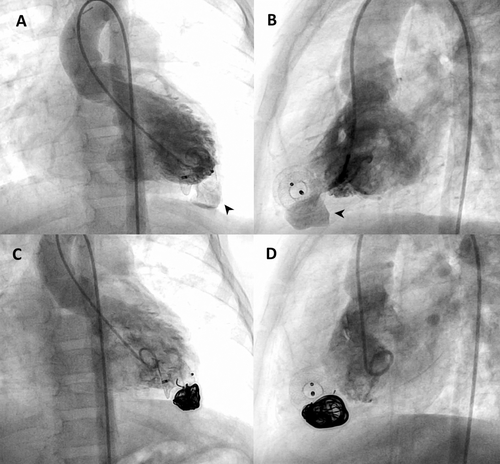
With informed consent and following a multidisciplinary discussion, an interventional closure procedure was performed. Baseline LV angiography revealed a 14 × 14 mm globular LVP with a narrow neck (neck-to-mouth ratio < 0.5), thin walls, and some areas with reduced contractility (Figure 4A,B, Supporting Information S3: Video 3). A 4 Fr Glide catheter and 2.7 Fr microcatheter were used to access the LVP, where a complex standard 0.2-inch 14 mm × 60 cm Ruby Framing Coil from Penumbra (Alameda, USA) was implanted to form a frame (Supporting Information S4: Video 4), followed by a Penumbra's 30 mm 0.02-inch shapeless packing coil to fully occlude the cavity. Final angiography confirmed LVP thrombo-exclusion (Figure 4C,D, Supporting Information S5: Video 5). At discharge, no residual shunt or pericardial effusion was noted, and at 12-month follow-up, the LVP remained closed (Supporting Information S6: Video 6). LV function and ECG were normal, and the child was on aspirin for 6 months.
3 Discussion
Pediatric LVOs, whether congenital or acquired, are now categorized based on LV geometry, wall thickness, and regional LVO wall motion [13]. Commonly, pseudoaneurysms typically lack normal myocardium, have a globular cavity, and a narrow neck (ratio 0.25–0.5), while aneurysms are thin-walled, dyskinetic, and feature a wide connection (ratio > 1) [1-3]. Rare and often underdiagnosed, particularly in older children and adults due to echocardiography's limitations, LVOs are usually asymptomatic but can lead to severe complications such as systemic embolization, heart failure, valvular regurgitation, rupture, ventricular tachycardia, or sudden death [1-3]. In this case, the asymptomatic LVP was incidentally detected during routine follow-up, having grown unnoticed to a detectable size. Increased awareness and regular monitoring could facilitate earlier diagnosis.
Acquired LVOs are mostly caused by myocardial infarction in adults, but they can also occur following cardiac surgery, trauma, or infection [1, 3, 12]. A few cases of iatrogenic LVOs have been identified during echocardiographic follow-up, primarily after perventricular VSD device closure [4-6, 8], with one case of cardiac erosion discovered incidentally during redo surgery following percutaneous VSD closure [7].
The exact pathogenesis of the LVO in this case remains uncertain. Exit angiography post-VSD closure showed no LVO but revealed that the left portion of the occluder was constrained, protruding into or possibly through the LV in an oval shape (Figure 1C,D, Supporting Information S1: Video 1). While this hypothesis cannot be dismissed, we doubt the device was protruding through the LV at the procedure's end (Supporting Information S1: Video 1). The implantation was smooth, with no signs of effusion or hemodynamic compromise. Although the constrained Nitinol device is theoretically unlikely to cause acute LV wall erosion, its implantation position could have been optimized with a smaller device. However, the chosen 10 mm device was not excessively oversized relative to the 8 mm MVSD needed for full shunt closure. Insufficient space in the apex of both ventricles prevented proper formation of the 18 mm retention disks (Supporting Information S1: Video 1). We hypothesize that the rigid Amplatzer muscular VSD device gradually reverted to its original memory shape, causing flattening of the left retention disk. This flattening may have exerted direct tension on the myocardium, weakening the LV wall and leading to cardiac erosion [6, 7]. This case underscores the risk of erosion when an Amplatzer device's disk is significantly constrained or deformed, while acknowledging the device's potential role in the complication.
Although less likely in our case and more commonly seen in perventricular muscular VSD closures, other mechanisms could include trauma to the LV-free wall from excessive wire manipulation or the advancement of the sheath and dilator over suboptimally positioned wires in the LV [4-6, 8]. Elevated LV pressure may have further contributed to the expansion of the eroded area, forming a pouch. Unlike typical aneurysms, this outpouching had a thin wall, narrow neck, and areas of abnormal contractility. MRI and LV angiography findings led to the diagnosis of an iatrogenic pediatric LVP linked to an erosion of the VSD closure device, which posed an elevated risk of rupture.
The rarity of LVOs in children complicates treatment due to the lack of standardized guidelines, with management tailored to clinical presentation, lesion size, and complication risk [1-3]. Surgery has been effective for narrow-based LVOs in children and neonates [4, 5, 7-9], while transcatheter interventions remain uncommon [6, 10-12]. In this case, the LVP's morphology favored interventional occlusion, with detachable coils selected to minimize tension from a Nitinol occluder, reduce wire manipulation and large sheath use, and lower the rupture risk from the aneurysm's thin walls.
4 Conclusion
Transcatheter device closure of muscular VSDs in small children is typically safe and effective, with rare, manageable complications. This case, however, underscores the risk of late, silent, and potentially fatal iatrogenic LVP from device erosion, successfully managed here percutaneously.
Ethics Statement
The procedure contributing to this work is by the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We obtained written informed consent from the patient's parents to use and publish their clinical data.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




