The everolimus eluting Synergy MegatronTM drug-eluting stent platform: Early outcomes from the European Synergy MegatronTM Implanters' Registry
Abstract
Background
The Synergy MegatronTM is an everolimus-drug eluting stent that may offer advantages in the treatment of aorto-ostial disease and large proximal vessels.
Aims
To report the short- to medium-term clinical outcomes from the European Synergy MegatronTM Implanters' Registry.
Methods
This registry was an investigator-initiated study conducted at 14 European centers. The primary outcome was target lesion failure (TLF), defined as the composite of cardiovascular death, target vessel myocardial infarction (MI), and target lesion revascularisation.
Results
Five hundred seventy-five patients underwent PCI with MegatronTM between 2019 and 2021. Patients were 69 ± 12 years old, 26% had diabetes mellitus, 24% had moderate-severe left ventricular impairment and 59% presented with an acute coronary syndrome. 15% were deemed prohibitively high risk for surgical revascularisation. The target vessel involved the left main stem in 55%, the ostium of the RCA in 13% and was a true bifurcation (Medina 1,1,1) in 50%. At 1 year, TLF was observed in 40 patients, with 26 (65%) occurring within the first 30 days. The cumulative incidence of TLF was 4.5% at 30 days and 8.6% (95% CI 6.3–11.7) at 1 year. The incidence of stent thrombosis was 0.5% with no late stent thromboses. By multivariate analysis, the strongest independent predictors of TLF were severe left ventricular impairment (HR 3.43, 95% CI: 1.67–6.76, p < 0.001) and a target vessel involving the left main (HR 4.00 95% CI 1.81–10.15 p = 0.001).
Conclusions
Use of the Synergy MegatronTM everolimus eluting stent in a ‘real-world’ setting shows favorable outcomes at 30 days and 1 year.
Abbreviations
-
- ACS
-
- Acute coronary syndrome
-
- LM
-
- Left main
-
- LSD
-
- Longitudinal stent deformation
-
- LV
-
- Left ventricular
-
- PCI
-
- Percutaneous coronary intervention
-
- STEMI
-
- ST-elevation myocardial infarction
-
- TLF
-
- Target lesion failure
1 INTRODUCTION
Percutaneous coronary intervention (PCI) is undertaken in increasingly high-risk patients and complex coronary disease, including aorto-ostial, left main (LM) and bifurcation disease.1, 2 There are fundamental differences in the arterial structure and composition of these lesions, versus those that are located in the rest of the coronary tree. A predominance of fibrotic and calcified tissue reduces arterial compliance3 and resists balloon dilatation, with chronic recoil contributing to increased rates of restenosis.4 Combined with the challenges of vessel eccentricity and angulation, PCI to these lesions is associated with inferior long term clinical outcomes.3, 5-7
The Synergy MegatronTM is an evolution of the Synergy abluminal coated, biosorbable polymer, everolimus-eluting, platinum chromium stent. Modifications versus the Synergy Large Vessel (4.0–5.0 mm nominal diameter) include more peaks (12 vs. 10), more connectors in the stent body (3 vs. 2), and increased strut thickness (89 vs. 81 µm) for a manufacturer reported increase in axial and radial strength on bench testing. A single platform allows overexpansion from 3.5 to 6.0 mm, such as when there is significant mismatch between the LM and its daughter vessels.8
However, whilst these features are theoretically attractive, the outcomes of the MegatronTM stent remain to be described outside of case reports and small studies.9-11 To address this, we report the early- to medium-term clinical and procedural outcomes from unselected, real-world patients, enrolled in a multicentre European registry.
2 METHODS
2.1 Study design and patient population
The European Synergy MegatronTM Implanters' Registry is an investigator initiated, retrospective, international, multicentre registry conducted at 14 centers in France, Ireland and the United Kingdom. Consecutive patients undergoing clinically indicated PCI between September 2019 and July 2021 with the MegatronTM stent were included in the registry. All patients received dual antiplatelet therapy for the duration of follow up in line with current ESC guidelines.12 The device manufacturer, Boston Scientific, had no role in study design, data analysis or control over manuscript publication.
Due to the retrospective nature of the study, patients were not approached for consent. All sites complied with local institutional review board and relevant national ethical requirements for the processing of fully anonymised data, which was transferred to King's College London, UK for final analysis.
2.2 Outcomes and definitions
The primary outcome for this study was target lesion failure (TLF), defined as cardiovascular death, target vessel myocardial infarction and target vessel revascularisation. The secondary outcome was patient-orientated composite events (POCE), defined as a composite of all-cause mortality, stroke, any myocardial infarction, and any revascularisation.
These endpoints, as well as target lesion revascularisation and stent thrombosis are as defined in the Academic Research Consortium (ARC)-2 consensus document.13 Procedural success was defined as the implantation of the MegatronTM stent without any complications that required additional percutaneous or surgical intervention.
Use of the MegatronTM was at the discretion of the operator. Given its indication for large proximal vessels, we stratified outcomes according to target lesion characteristics. Complex lesions were defined as those involving the left main, the ostium of the right coronary artery, or bifurcations. The remaining lesions were defined as noncomplex.
2.3 Statistical analysis
Continuous variables are presented as mean ± standard deviation and compared using the Student's unpaired t-test. Categorical variables are presented as counts and percentages (of available data when incomplete). They were compared using the Mann-Whitney, or Fisher's exact tests as appropriate. Cumulative event rates were calculated using the Kaplan-Meier method and comparisons made between groups using the log-rank test. Predictors of TLF were identified by Cox proportional hazards analysis. Covariates were first screened in univariate models and those that were both significant (p-value < 0.10) and judged to be clinically important were included in a multivariate analysis. This was used to estimate hazard ratios (HR) and 95% confidence intervals (CI). A probability value of <0.05 was considered significant. All data were analyzed using GraphPad Prism 9.5.1 (GraphPad Software, CA, USA).
3 RESULTS
Five hundred seventy-five patients underwent PCI with MegatronTM during the study period. Clinical outcomes were available at a median of 365 days (IQR 30-365) with 30-day and 1-year follow up available in 100% and 58%, respectively. Baseline characteristics are detailed in Table 1 and stratified by target lesion complexity in Table S1. The mean age was 68.5 ± 11.5 years and 83% were male. Sixty-two percent were hypertensive, 26% were diabetic and 41% had a history of previous percutaneous or surgical revascularisation. 42% had left ventricular impairment and 59% presented with an acute coronary syndrome (ACS). Of those presenting with ACS, 34% were STEMI and 57% involved intervention to the LM.
| N = 575 | |
|---|---|
| Age (years) | 68.5 ± 11.5 |
| Male | 456 (83) |
| Hypertension | 341 (62) |
| Dyslipidaemia | 328 (60) |
| Diabetes | 142 (26) |
| End Stage Renal Failure | 19 (3) |
| Previous MI | 139 (25) |
| Previous PCI | 174 (32) |
| Previous CABG | 50 (9) |
| Previous CVA | 57 (10) |
| PVD | 55 (10) |
| BMI | 28.0 ± 6.9 |
| LV function | |
| Normal | 313 (58) |
| Mild-Moderate impairment | 173 (32) |
| Severe impairment | 54 (10) |
| Clinical Syndrome | |
| Stable angina | 223 (41) |
| ACS - NSTEMI/UA | 215 (39) |
| ACS - STEMI | 112 (20) |
- Abbreviations: BMI, body mass index; CABG, coronary artery bypass graft; CVA, cerebrovascular accident; MI, myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST elevation myocardial infarction.
Procedural characteristics are described in Table 2. The target vessel for the MegatronTM stent involved the LM in 55% and the ostium of the RCA in 13%. Fifty percent of targets were bifurcation lesions (93% of these LM bifurcation). Intravascular imaging was used in 78% of all LM cases, with an overall rate of 65% within the whole cohort. Procedural success was achieved in 98%.
| N = 575 | |
|---|---|
| Surgical turndown | 82 (15) |
| Radial access | 496 (91) |
| Target vessel | |
| Isolated left main | 27 (5) |
| Left main and LAD/LCx | 290 (50) |
| Ostial RCA | 75 (13) |
| Saphenous vein graft | 6 (1) |
| Chronic total occlusion | 45 (8) |
| Bifurcation | 286 (50) |
| Provisional | 202 (35) |
| Upfront 2 stent | 84 (15) |
| For in-stent restenosis | 35 (6) |
| Number of stents | 1.9 ± 1.1 |
| Stented segment length | 46.9 ± 36.0 |
| Intravascular imaging | 359 (65) |
| IVUS | 325 (59) |
| OCT | 34 (6) |
| Post dilatation | 478 (87) |
| Procedural success | 562 (98) |
- Abbreviations: IVUS, intravascular ultrasound; OCT, optical coherence tomography.
3.1 Clinical outcomes
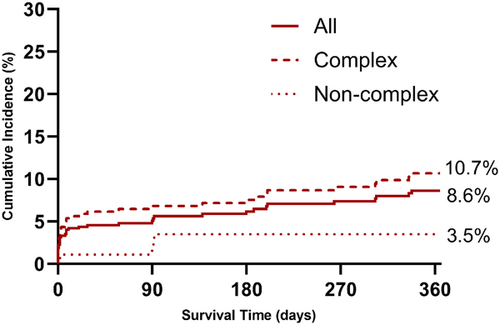
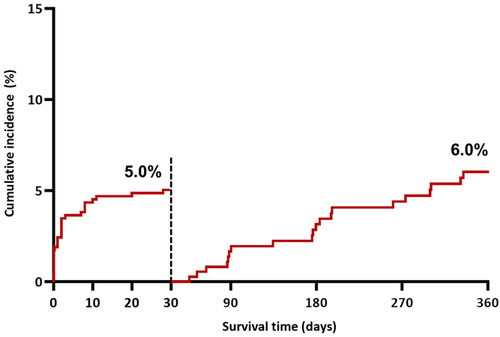
TLF occurred in 40 of 575 patients at a median of 8 (IQR 0.1–127) days after MegatronTM implantation. The cumulative incidence of TLF by the Kalpan-Meier method was 8.6% (95% CI 6.3–11.7) at 1 year. TLF was higher with complex versus noncomplex target lesions (10.7% vs. 3.5%, p = 0.008) (Figure 1). Landmark analysis shows that 26 of the 40 (65%) events, occurred within 30 days of the procedure, corresponding to an event rate of 4.7% (Figure 2). The cumulative incidence of POCE was 4.9% at 30 days and 10.6% (95% CI 8.0–13.4) at 1 year.
Early TLF was driven by cardiovascular death which occurred in in 17 (65%) patients. Myocardial infarction occurred in the remaining nine (35%) patients, six of which were periprocedural. The remaining target vessel myocardial infarctions included two acute, and one subacute stent thromboses. One of these occurred within a MegatronTM stent. This was an acute thrombosis within a stent that had been placed across the LM-LAD bifurcation during primary PCI for STEMI in a patient with severe LV dysfunction. After 30 days, cardiovascular death occurred in a further six patients and spontaneous target vessel myocardial infarction in five patients. Target lesion revascularisation occurred in seven patients. There were no cases of late stent thrombosis.
Patients who experienced TLF were more likely to be diabetic with a history of MI, and severe impairment of LV function. (Table 3). They were more likely to have undergone PCI that involved the left main or for in stent restenosis (Table 4).
| TLF (N = 40) | No TLF (N = 535) | p-value | |
|---|---|---|---|
| Age (years) | 70.8 ± 9.0 | 67.2 ± 11.6 | 0.105 |
| Male | 32 (80) | 424 (83) | 0.612 |
| Hypertension | 25 (63) | 316 (62) | 0.999 |
| Dyslipidaemia | 23 (58) | 305 (60) | 0.867 |
| Diabetes | 16 (40) | 126 (25) | 0.040* |
| End Stage Renal Failure | 3 (8) | 16 (3) | 0.153 |
| Previous MI | 17 (43) | 122 (24) | 0.014* |
| Previous PCI | 14 (35) | 160 (31) | 0.724 |
| Previous CABG | 6 (15) | 44 (9) | 0.245 |
| Previous CVA | 7 (18) | 50 (10) | 0.245 |
| PVD | 5 (13) | 50 (10) | 0.582 |
| BMI | 25.8 ± 8.5 | 28.1 ± 6.8 | 0.054 |
| LV function | |||
| Normal | 17 (43) | 296 (59) | 0.101 |
| Mild-Moderate impairment | 11 (28) | 161 (32) | 0.859 |
| Severe impairment | 12 (30) | 43 (9) | <0.001* |
| Clinical Syndrome | |||
| Stable angina | 12 (30) | 211 (41) | 0.158 |
| ACS - NSTEMI/UA | 15 (38) | 200 (39) | 0.868 |
| ACS - STEMI | 13 (33) | 99 (19) | 0.064 |
- Note: Percentages may not total 100% due to rounding and counts may be less than the total participants in cases of incomplete data.
- Abbreviations: BMI, body mass index; CABG, coronary artery bypass graft; CVA, cerebrovascular accident; MI, myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST elevation myocardial infarction.
| TLF (N = 40) | No TLF (N = 535) | p-value | |
|---|---|---|---|
| Surgical turndown | 10 (26) | 72 (14) | 0.063 |
| Radial access | 32 (82) | 464 (91) | 0.082 |
| Target vessel | |||
| Isolated left main | 2 (5) | 25 (5) | 0.925 |
| Left main and LAD/LCx | 33 (83) | 284 (53) | <0.001* |
| Ostial RCA | 3 (8) | 72 (13) | 0.462 |
| Saphenous vein graft | 1 (3) | 5 (1) | 0.353 |
| Chronic total occlusion | 2 (5) | 43 (8) | 0.762 |
| Bifurcation | |||
| Provisional | 19 (48) | 183 (34) | 0.121 |
| Upfront 2 stent | 10 (25) | 74 (14) | 0.063 |
| For in-stent restenosis | 6 (15) | 29 (6) | 0.030* |
| Number of stents | 2 (1-3) | 2 (1-3) | 0.158 |
| Stent length | 54.1 ± 49.5 | 46.3 ± 34.7 | 0.189 |
| Intravascular imaging | |||
| IVUS | 27 (68) | 298 (58) | 0.317 |
| OCT | 2 (5) | 32 (6) | 0.999 |
| Post dilatation | 38 (95) | 439 (86) | 0.145 |
- Note: Percentages may not total 100% due to rounding and counts may be less than the total participants in cases of incomplete data.
- Abbreviations: IVUS, intravascular ultrasound; OCT, optical coherence tomography.
After multivariate analysis, previous MI, severe LV impairment, STEMI, target vessel involving the left main stem, and PCI for in stent restenosis were independently associated with an increased risk of TLF at 1 year (Table 5).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Diabetes | 1.94 (1.01–3.62) | 0.040 | 1.54 (0.78–2.92) | 0.195 |
| Previous MI | 2.23 (1.17–4.15) | 0.012 | 2.34 (1.17–4.61) | 0.015* |
| Severe LV impairment | 4.02 (1.97–7.72) | <0.001 | 3.45 (1.67–6.76) | <0.001* |
| STEMI | 1.93 (0.97–3.67) | 0.051 | 2.82 (1.36–5.57) | 0.004* |
| Target involving LM23, 24 | 3.65 (1.71–9.00) | <0.002 | 4.00 (1.81–10.15) | 0.001* |
| For ISR | 2.68 (1.01–5.95) | 0.026 | 2.72 (0.97–6.51) | 0.036* |
- Abbreviations: CVA, cerebrovascular accident; ISR, in stent restenosis; LAD, left anterior descending; LCx, left circumflex; LM, left main; LV, left ventricular; STEMI, ST elevation myocardial infarction.
- * Significant with p < 0.05 after multivariate analysis.
4 DISCUSSION
Use of the Synergy MegatronTM, in a complex and real-world population is associated with acceptable short term clinical outcomes at 30 days and 1 year. To our knowledge, this is the largest study reporting on the clinical outcomes of this platform. The factors independently associated with TLF in this study are well established predictors of poor short-term outcomes.14-16
Aorto-ostial lesions present several obstacles that must be overcome during PCI. Eccentricity and the “funnel” shape of the LM, combined with the fundamental limitations of two-dimensional angiography contribute to high rates of geographical miss in these lesions.17 The distal left main stem involves the largest coronary bifurcation of all, and two further ostia. These share the histological features of fibrosis and calcification as the aorto-ostial junction, but may also be combined with severe angulation and a large mismatch in size, increasing the PCI technical complexity.6 Despite this, it is the ostium of the right coronary that appears to be associated with the highest rates of TLF, with stent fracture identified in half of those presenting with ISR, in one series.18
Contemporary thin strut drug-eluting stents whilst highly deliverable, are used in all coronary segments without regard for these differences, however. The Synergy Megatron is designed to address these specific issues. The increased radial and axial strength of this platform may resist vessel recoil as well as longitudinal stent deformation (LSD). LM intervention significantly increases the risk of LSD,19 which was observed in 6.6% of final IVUS images from the EXCEL trial. This was associated with an increase in cardiac death, MI, and ischemia driven target lesion revascularisation at 3 years (28.3% vs. 13.9%, p = 0.02) as well as numerically greater stent thrombosis, regardless of stent area or protrusion into the aorta.20 Correct use of the proximal optimization technique (POT) is mandatory to appose the stent within the proximal main vessel, facilitate entry into the side branch, and to avoid disruption to the neocarina. However, the reduction of metal and eluted drug to vessel ratio from the POT has also been suggested as a potential source of long-term device failure.21, 22 The Megatron's single platform allows over-expansion to 6 mm, suited to adequately scaffolding large proximal vessels despite any size mismatch between the LM and the left anterior descending (LAD) or circumflex (LCx) arteries.
Given the Megatron'sTM indication for aorto-ostial, left main bifurcation, and proximal disease, the results are perhaps best considered in comparison to the 30-day and 1-year MACE outcomes from the PCI arms of NOBLE (4% and 8%) and EXCEL (5% and 8%)23, 24; the 1 year composite primary outcome of EBC MAIN (14.7% within the stepwise provisional group)25; as well as a recent left main analysis of the Swedish Coronary Angiography and Angioplasty (SCAAR) registry.1 In common with the SCAAR registry, which reported a 14% and 24% MACE rate, with a 7.9% rate of periprocedural complications, this study included surgical turndown (15%), with a high proportion of co-morbidities and acute coronary syndromes. Compared to these studies, the 30-day TLF rate of 4.7%, 1 year rate of 8.6%, and periprocedural complication rate of 2.3% in this study are highly favorable. We show that the majority of events occur early, particularly within the first 10 days. This is therefore likely to reflect the underlying presentation, rather than the effect of any treatment strategy or device such as the MegatronTM.26-29 The early stent thrombosis rate within the whole cohort was 0.5%, similar to the 0.2%–0.6% reported in other studies.23, 24, 29 These results should perhaps be considered unsurprising given that the MegatronTM is an iteration of the Synergy platform, with an established safety record.30-32 Nonetheless, it is seems probable that the purported resistance to recoil and deformation of this new platform will only yield detectable clinical benefits with long term follow up.
Although these indirect comparisons with existing data are hypothesis generating, true differences in meaningful clinical outcomes can only be assessed in the context of a prospective randomized trial.
4.1 Limitations
This study has limitations that are inherent to all observational registries. Although consecutive patients have been enrolled at each centre, the decision to use a MegatronTM was at the operator's discretion, introducing selection bias. Although we consider the reporting of all cause death to be robust, adjudication of cardiovascular versus non-cardiovascular death was not performed by a clinical events committee. Assessment of periprocedural myocardial infarction was not mandated by protocol. Measures of procedural complexity such as the use of calcium modification devices and anatomical SYNTAX score were not available as these are not routinely collected across all national registries for the participating centers. These findings should be considered most robust for short term outcomes, given the loss to follow up at 1 year. Finally, although this is the largest published series to date, the numbers are nonetheless relatively small.
5 CONCLUSIONS
We report the largest real-world registry providing evidence of the safety and clinical performance of MegatronTM, in a real-world, complex patient population, with satisfactory 30-day and 1-year clinical outcomes. Additional prospective, randomized data is required to further assess this novel stent platform in the setting of aorto-ostial coronary disease.
CONFLICT OF INTEREST STATEMENT
KDS and ML are supported by the British Heart Foundation (RE/18/2/34213, FS/CRTF/22/24342). MM, JCS, and FD report honoraria, speaker, or consultancy fees from Boston Scientific. SJW is currently an employee of Boston Scientific. The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.