A preliminary study of minimal left atrial appendage occlusion using Watchman under the guidance of fluoroscopy
Abstract
Background
Left atrial appendage occlusion (LAAO) has been considered an alternative treatment to prevent embolic stroke in patients with nonvalvular atrial fibrillation (NVAF). However, it carries a risk of general anesthesia or esophageal injury if guided by transesophageal echocardiography (TEE).
Aims
We aimed to investigate the feasibility and safety of minimal LAAO (MLAAO) using Watchman under fluoroscopy guidance alone in patients with NVAF.
Methods
A total of 249 consecutive patients with NVAF who underwent LAAO using the WATCHMAN device were divided into two groups: the Standard LAAO (SLAAO) group and the MLAAO group. Procedural characteristics and follow-up results were compared between the two groups.
Results
There was no statistically significant difference in the rate of successful device implantation (p > 0.05). Fluoroscopy time, radiation exposure dose, and contrast medium usage in the MLAAO group were higher than those in the SLAAO group (p < 0.001). The procedure time and hospitalization duration were significantly lower in the MLAAO group than those in the SLAAO group (p < 0.001). The occluder compression ratio, measured with fluoroscopy, was lower than that measured with TEE (17.63 ± 3.75% vs. 21.69 ± 4.26%, p < 0.001). Significant differences were observed between the SLAAO group and the MLAAO group (p < 0.05) in terms of oropharyngeal/esophageal injury, hypotension, and dysphagia. At 3 months after LAAO, the MLAAO group had a higher incidence of residual flow within 1−5 mm compared to the SLAAO group, although the difference was not statistically significant.
Conclusion
MLAAO guided by fluoroscopy, instead of TEE, without general anesthesia simplifies the operational process and may be considered safe, effective, and feasible, especially for individuals who are unable to tolerate or unwilling to undergo TEE or general anesthesia.
1 INTRODUCTION
The left atrial appendage (LAA) is a highly variable blind-end cystic structure composed of pectinate muscle (comb muscle) and trabecula. An increasing number of studies have demonstrated that the morphology of the LAA is closely associated with the risk of stroke, with more than 90% of thrombosis occurring in the LAA among patients with nonvalvular atrial fibrillation (NVAF).1, 2 Currently, left atrial appendage occlusion (LAAO) is considered an ideal alternative treatment for preventing embolic stroke in patients with NVAF.3 Standard LAAO (SLAAO) typically involves the use of X-rays, general anesthesia, endotracheal intubation, mechanical ventilation and transesophageal echocardiographic (TEE) guidance, especially in those centers at the beginning of their experience. In some cases, however, SLAAO may increase the risks associated with anesthesia and esophageal injury, leading to delayed patient recovery, prolonged procedure time, increased costs, and extended hospitalization. In addition, SLAAO guided by TEE is not feasible for patients with gastrointestinal or stomatological diseases. If fluoroscopic assessment can replace TEE guidance, it would significantly simplify the procedure, reduce complications related to TEE, intubation, general anesthesia, lower operation costs, and shorten both the procedure and postoperative recovery time. Emerging evidence suggests that LAAO guided by fluoroscopy guidance only (minimal LAAO [MLAAO]) is also safe and reliable.4-7 The aim of this study was to summarize the preliminary results of MLAAO in patients with NVAF under the guidance of fluoroscopy at our center, assess the feasibility and safety of MLAAO and explore its potential benefits.
2 METHODS
A total of 254 consecutive patients diagnosed with NVAF who were scheduled to undergo LAAO using the WATCHMAN™ device (Boston Scientific) at Taizhou People's Hospital affiliated with Nanjing Medical University from January 2019 to October 2021 were initially included in the study. Five patients were excluded: three due to the presence of LAA thrombus, and two due to an excessively large LAA. Consequently, a total of 249 patients were retrospectively analyzed, consisting of 148 males and 101 females, aged between 58 and 86 years, with an average age of 69.61 ± 5.23 years. The selection of either the SLAAO or MLAAO approach was based on informed consents made by the patients. Ultimately, 183 patients were assigned to the SLAAO group, while 66 patients were assigned to the MLAAO group. All patients met the following inclusion criteria: age > 18 years, NVAF, high risk for stroke (CHA2DS2-VASC score ≥ 2 for males and ≥3 for females), intolerance to long-term oral anticoagulant therapy, contraindications to anticoagulation, or oral anticoagulant therapy failure (e.g., stroke or transient ischemic attack during anticoagulant therapy). The study was approved by the Ethics Committee of Taizhou People's Hospital affiliated with Nanjing Medical University, and written informed consent was obtained from all patients before the procedure. The procedure was performed by two physicians with a minimum of 40 prior LAAO procedure volumes. Successful implantation was defined as stable device positioning with no or residual peri-device leak (PDL) width <5 mm. Furthermore, the analysis of digital subtraction angiography (DSA) data during the procedure in both groups was performed by a specialized engineer using Siemens DSA software.
2.1 CT angiography (CTA) examination for preprocedural and follow-up imaging
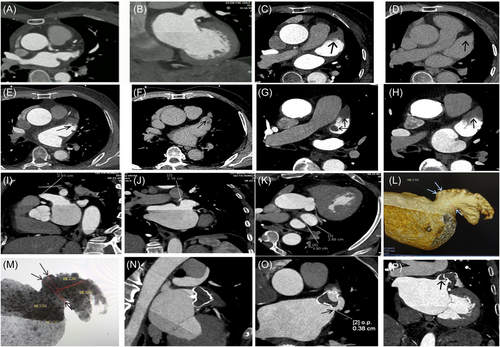
CTA with dual-source CT (Siemens SOMATOM force) was performed 24 h before LAAO to exclude the presence of thrombus in the left atrium (LA) or LAA, and to measure the ostial dimensions and depths of the LAA. Additionally, routine CTA was performed at 3 months postprocedure for all patients. In brief, 80 mL of nonionic contrast agent (Iomeron 350) was injected at a rate of 4−5 mL/s. The scanning parameters were as follows: voltage 100-120 kV, effective tube current 800−1235 Ma, collimator width 256 × 0.625 mm, frame rotation time 270 ms, and spacing 0.2. A two-phase scan was performed, with the second scan delayed by 60 s from the first phase scan. Thrombosis was ruled out if no low-density filling defects were observed during the dual-phase scanning. The presence of blood stasis without thrombosis was defined as the appearance of a filling defect in the first phase that disappeared in the second phase after the 60-second delay. The presence of thrombus was defined as a low-density filling defect observed in the first phase, and persisted in the second phase. When the scan was completed, the data were transferred to the Syngo VB10 workstation (Siemens), and image analysis was performed using MM reading software, including three-dimensional reconstruction (3DR), volume rendering (VR), and maximum intensity projection (MIP), to obtain multi-directional anatomical information of the LAA and Watchman occluder images (Figure 1).
2.2 SLAAO procedure using the Watchman device
An SLAAO procedure was performed according to previously described methods.8 In brief, the procedure involved the administration of general anesthesia and TEE guidance. Following a successful transseptal puncture, DSA of the LAA was performed. Based on the measured parameters of LAA diameter and depth from TEE, CTA, and DSA, a Watchman device of LAA with an oversized diameter of 4–6 mm was selected and released according to the PASS principle.
2.3 MLAAO procedure using the watchman device
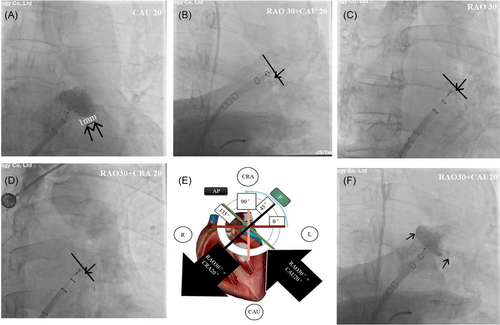
In regard to MLAAO, the procedure was performed with local infiltration anesthesia of 1% lidocaine. The transseptal puncture site was determined as inferior−posterior based on the fluorescence image of the anterior−posterior position and right anterior oblique (RAO) 45°. Following a successful transseptal puncture, contrast angiography was performed in three positions, RAO 30°/caudal (CAU) 20°, RAO 30°, and RAO 30°/cranial (CRA) 20°, using a sheath and pigtail catheter to visualize the morphology of the LAA. The largest LAA orifice diameter, measured with calibration of the marker on the delivery sheath in the three views, was considered the final diameter of the landing zone. Based on the measured LAA diameter from CTA and DSA, a Watchman device with an oversized diameter of 4–6 mm was selected and deployed under fluoroscopic guidance. It should be noted that a previous study indicated that the ostium diameter and depth of the LAA measured by CTA were larger than those measured by DSA [8]. In case of discrepancy between DSA and CT scan results, a larger-sized occluder could be considered on the basis of the DSA measurement results and the principle of 4-6 mm degree of device over-sizing. The “PASS” principle of fluoroscopy was then employed to assess (Figure 2): “P” (Position)—a tangential CAU 20°/RAO 0°−45° view was used to assess the exposure of the occluder's shoulder, ensuring it was located at the LAA ostium or slightly away (over-shoulder distance within 1/4–1/3 of the diameter of the Watchman device); “A” (Anchor)—stable anchoring was confirmed by a tug test without displacement under fluoroscopy; “S” (Size)—the occluder compression ratio was determined at the widest cross-section using tangent fluoroscopic views from three positions: RAO 30°/CAU 20° (equal to TEE 135°), RAO 30° (equal to TEE 90°), and RAO 30°/CRA 20° (equal to TEE 135°), and calibrated with the marker on the delivery sheath. The compression rate was then calculated as: (manufacturer device diameter—measured diameter by DSA)/manufacturer device diameter * 100%. A value between 8% and 25% was deemed acceptable; “S” (Seal)—the degree of sealing was evaluated by examining PDL in three fluoroscopic views: RAO 30°/CAU 20°, RAO 30°, and RAO 30°/CRA 20°. A result with no leakage or residual leakage < 5 mm were considered acceptable. If the “PASS” principle was satisfied, the Watchman device was then released. Following the procedure, transthoracic echocardiography (TTE) was performed to detect the presence of pericardial effusion.
2.4 Transseptal puncture in SLAAO and MLAAO procedures
In the SLAAO procedure, the targeted transseptal puncture site for an inferior-posterior location was determined using four-chamber (vertical) and short-axis (anterior-posterior) views on TEE. In the MLAAO procedure, the optimal transseptal puncture site for an inferior-posterior location was obtained through the following steps: first, the sheath tip was positioned at a height of half to one vertebral body above the lower edge of the left atrial shadow in the posteroanterior position. Then, the sheath tip was adjusted to the anterior half of the posterior border of the left atrium, at one vertebral body in RAO 45°. Under fluoroscopy guidance, the puncture stylet was slowly advanced to puncture the interatrial septum, and a small amount of contrast agent was injected to visualize the left atrium to confirm the entry of the needle tip into the left atrium.
2.5 Follow-up
Follow-up assessments were performed for all patients at 3 months after LAAO. During the follow-up period, adverse events such as thromboembolic events, bleeding events, and mortality were documented. Residual leakage and device-related thrombus (DRT) formation were evaluated using CTA. Generally, a treatment plan involving anticoagulation combined with antiplatelet therapy (aspirin or clopidogrel) was recommended for the initial 3 months following LAAO. Subsequently, dual antiplatelet therapy (DAT) consisting of both aspirin and clopidogrel was administered for an additional 3 months. Thereafter, life-long administration of aspirin or clopidogrel was recommended if there were no persistent PDLs greater than 5 mm and no device-related thrombosis detected by CTA during the follow-up period.
2.6 Statistical analysis
The experimental data were analyzed using the statistical software SPSS 26.0. The normality of distribution for continuous variables was assessed using the Shapiro−Wilk test, and these variables were reported as mean ± standard deviation. Comparisons between groups were performed using paired sample t-test or Mann–Whitney U test, as appropriate. Categorical data were presented as counts and percentages, and analyzed using a χ2 test. Pearson's correlation analysis was performed to examine the correlation between device compression rates measured by TEE and fluoroscopy. A p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Comparison of baseline characteristics between the SLAAO group and the MLAAO group
Out of the 183 patients initially selected for the SLAAO group, two patients were transferred to the MLAAO group due to their inability to undergo TEE. Similarly, out of the 66 patients initially selected for the MLAAO group, one patient was transferred to the SLAAO group due to nervousness. Consequently, 182 patients underwent SLAAO and 67 patients underwent MLAAO, all of whom successfully completed the LAAO procedure and long-term follow-ups. The baseline characteristics of the two groups are shown in Table 1. There was a statistically significant difference in age between the SLAAO and MLAAO groups (p = 0.000). However, all other characteristics were similar between the two groups (p > 0.05, respectively).
| Patient characteristics | SLAAO group (n = 182) | MLAAO group (n = 67) | t/χ2 value | p Value |
|---|---|---|---|---|
| Age, years | 68.76 ± 4.98 | 72.16 ± 5.23 | 4.76 | 0.000 |
| Female, n (%) | 86 (47.25) | 35 (52.24) | 0.49 | 0.484 |
| Body mass index, kg/m2 | 23.68 ± 3.04 | 23.41 ± 3.01 | 0.63 | 0.265 |
| Coronary artery disease, n (%) | 40 (21.98) | 15 (22.38) | 0.01 | 0.920 |
| Hypertension, n (%) | 94 (51.65) | 36 (53.73) | 0.00 | 1.000 |
| Diabetes mellitus, n (%) | 46 (25.27) | 19 (28.36) | 0.24 | 0.624 |
| Vascular disease, n (%) | 39 (21.43) | 19 (28.36) | 1.32 | 0.251 |
| Stroke/TIA/thromboembolism, n (%) | 61 (33.52) | 26 (38.81) | 0.60 | 0.439 |
| Major bleeding history (%) | 20 (10.99) | 7 (10.45) | 0.02 | 0.888 |
| Congestive heart failure, n (%) | 27 (14.84) | 15 (22.39) | 1.99 | 0.158 |
| Abnormal liver function, n (%) | 9 (4.95) | 5 (7.46) | 0.59 | 0.442 |
| Abnormal renal function, n (%) | 36 (19.78) | 8 (11.94) | 2.07 | 0.150 |
| Alcohol, n (%) | 21 (11.54) | 10 (14.93) | 0.02 | 0.888 |
| CHA2DS2-VASc | 3.01 ± 0.96 | 2.95 ± 1.28 | 0.39 | 0.348 |
| HAS-BLED | 2.68 ± 0.71 | 2.52 ± 0.89 | 1.47 | 0.071 |
| Paroxysmal atrial fibrillation, n (%) | 28 (15.39) | 12 (17.91) | 0.23 | 0.632 |
| Persistent atrial fibrillation, n (%) | 154 (84.61) | 55 (82.09) | 0.23 | 0.632 |
| Left ventricular ejection fraction, % | 61.26 ± 5.34 | 60.92 ± 7.71 | 0.39 | 0.348 |
- Abbreviations: MLAAO, minimal left atrial appendage occlusion; SLAAO, standard left atrial appendage occlusion.
3.2 Comparison of procedural characteristics between the SLAAO group and the MLAAO group
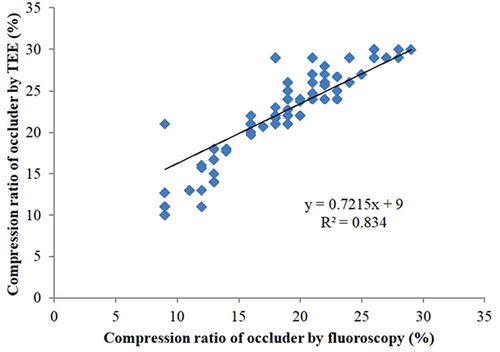
As shown in Table 2, there was no statistically significant difference in the rate of successful device implantation between the SLAAO group and the MLAAO group (98.91% vs. 98.485, p > 0.05). Furthermore, no statistically significant differences were observed in device resizing, deployment attempts per procedure, device size, the incidence of residual flow, and occluder shoulder exposure between the two groups (p > 0.05, respectively). However, the MLAAO group exhibited higher fluoroscopy time, radiation exposure dose, and contrast medium compared to those in the SLAAO group (p < 0.001, respectively). Moreover, the MLAAO group demonstrated significantly shorter procedure time and hospitalization duration compared to the SLAAO group (p < 0.001, respectively). Regarding the occluder compression ratio, a t-test was utilized to compare the values measured by TEE and fluoroscopy, and the correlation between TEE and fluoroscopy measurements were analyzed using a Spearman analysis in the SLAAO group. As presented in Table 2 and Figure 3, the occluder compression ratio measured with fluoroscopy was lower than that measured with TEE (t = 8.61, p < 0.001), but a good correlation (R2 = 0.834) was observed. Consequently, there was no significant difference in the occluder compression ratio measured with fluoroscopy between the SLAAO group and the MLAAO group. However, when comparing the occluder compression ratio measured with fluoroscopy in the MLAAO group to that measured with TEE in the SLAAO group, the MLAAO group exhibited a lower rate (17.63 ± 3.75% vs. 21.69 ± 4.26%, p < 0.001). Significant differences were observed between the SLAAO group and the MLAAO group in terms of oropharyngeal/esophageal injury, hypotension, and dysphagia (p < 0.05).
| Procedural parameters | SLAAO group (n = 182) | MLAAO group (n = 67) | t/χ2 value | p Value |
|---|---|---|---|---|
| Implantation success rate, % | 98.91 | 98.48 | 0.00 | 1.000 |
| Device resizing, n (%) | 3 (1.65) | 1 (1.49) | 0.00 | 1.000 |
| Deployment attempts per procedure | 1.14 ± 0.42 | 1.20 ± 0.48 | 0.96 | 0.169 |
| Procedure time, min | 57.51 ± 7.08 | 37.48 ± 6.89 | 19.95 | 0.000 |
| Fluoroscopy time, min | 10.64 ± 2.08 | 12.85 ± 2.12 | 7.39 | 0.000 |
| Radiation exposure dose, mGy | 617.52 ± 19.78 | 667.85 ± 23.29 | 16.95 | 0.000 |
| Contrast medium, mL | 41.96 ± 7.27 | 89.25 ± 6.19 | 47.29 | 0.000 |
| Hospital stay, day | 6.22 ± 0.49 | 5.14 ± 0.42 | 16.02 | 0.000 |
| LAA morphology | ||||
| Cauliflower, n (%) | 116 (63.73) | 41 (61.19) | 0.14 | 0.708 |
| Chicken wing, n (%) | 29 (15.93) | 12 (17.91) | 0.23 | 0.632 |
| Cactus, n (%) | 24 (13.19) | 11 (16.42) | 0.42 | 0.517 |
| Wind stock, n (%) | 13 (7.14) | 3 (4.48) | 0.58 | 0.447 |
| Device size | ||||
| 21 mm, n (%) | 12 (6.59) | 5 (7.46) | 0.06 | 0.806 |
| 24 mm, n (%) | 33 (18.13) | 12 (17.91) | 0.00 | 1.000 |
| 27 mm, n (%) | 57 (31.32) | 21 (31.34) | 0.00 | 1.000 |
| 30 mm, n (%) | 34 (18.68) | 10 (14.93) | 0.47 | 0.493 |
| 33 mm, n (%) | 46 (25.27) | 19 (28.36) | 0.24 | 0.624 |
| Residual flow | ||||
| 0 mm, n (%) | 129 (70.88) | 45 (67.16) | 0.32 | 0.572 |
| 1−5 mm, n (%) | 53 (29.12) | 22 (32.84) | 0.32 | 0.572 |
| ≥5 mm, n (%) | 0 (0.00) | 0 (0.00) | 0 | 1.000 |
| Exposure shoulder of occluders | ||||
| Yes, n (%) | 87 (47.80) | 34 (50.75) | 0.17 | 0.680 |
| No, n (%) | 95 (52.20) | 33 (49.25) | 0.17 | 0.680 |
| Compression ratio of occluders | ||||
| DSA, % | 17.92 ± 4.09 | 17.63 ± 3.75 | 0.51 | 0.305 |
| TEE, % | 21.69 ± 4.26 | — | ||
| Complications | ||||
| Pericardial effusion, n (%) | 2 (1.10) | 1 (1.49) | 0.06 | 0.807 |
| Cardiac tamponade, n (%) | 1 (0.55) | 0 (0.00) | 0.62 | 0.431 |
| Vagal reflex, n (%) | 0 (0.00) | 1 (1.49) | 1.10 | 0.294 |
| Oropharyngeal/Esophageal injury, n (%) | 10 (5.49) | 0 (0.00) | 2.11 | 0.146 |
| Oropharyngeal hematoma, n (%) | 3 (1.65) | 0 (0.00) | 1.12 | 0.289 |
| Hypotension, n (%) | 11 (6.04) | 1 (1.49) | 2.21 | 0.137 |
| Dysphagia, n (%) | 6 (3.30) | 0 (0.00) | 2.26 | 0.133 |
| Fever, n (%) | 3 (1.65) | 1 (1.49) | 0.01 | 0.920 |
| Vascular complications, n (%) | 2 (1.10) | 1 (1.49) | 0.06 | 0.807 |
- Abbreviations: MLAAO, minimal left atrial appendage occlusion; SLAAO, standard left atrial appendage occlusion.
3.3 Comparison of follow-up results 3 months after LAAO between the SLAAO and MLAAO groups
Follow-up CTA was performed in all patients at 3 months after LAAO, confirming the proper placement of the Watchman devices. There were 138 (75.82%) patients without any residual flow in the SLAAO group, while there were 46 (68.66%) in MLAAO group, (p > 0.05). Although not statistically significant, the incidence of residual flow within 1–5 mm was higher in the MLAAO group (29.85%) compared to the SLAAO group (24.18%). Additionally, one case in the MLAAO group exhibited residual flow greater than 5 mm. DRT occurred in three patients at 3 months after the procedure, with two cases (1.09%) in the SLAAO group and one case (1.49%) in the MLAAO group. Similarly, one case (0.55%) of stroke was noted in the SLAAO group, while bleeding complications were observed in five cases at 3 months after operation, with three cases (1.65%) in the SLAAO group and two cases (2.98%) in the MLAAO group. The detailed results are presented in Table 3.
| Variable | SLAAO group (n = 182) | MLAAO group (n = 67) | t/χ2 value | p Value |
|---|---|---|---|---|
| Occluder displacement, n (%) | 0 (0) | 0 (0) | 0 | 1.000 |
| Residual flow, n (%) | ||||
| 0 mm, n (%) | 138 (75.82) | 46 (68.66) | 1.30 | 0.254 |
| 1−5 mm, n (%) | 44 (24.18) | 20 (29.85) | 0.83 | 0.362 |
| ≥5 mm, n (%) | 0 (0) | 1 (1.49) | 1.10 | 0.294 |
| Device-related thrombus, n (%) | 2 (1.09) | 1 (1.49) | 0.06 | 0.807 |
| Stroke, n (%) | 1 (0.55) | 0 (0) | 0.37 | 0.543 |
| Bleeding, n (%) | 3 (1.65) | 2 (2.98) | 0.44 | 0.507 |
| Postprocedure medications | ||||
| Warfarin, n (%) | 27 (14.84) | 9 (13.43) | 0.08 | 0.777 |
| Dabigatran, n (%) | 13 (7.14) | 4 (5.97) | 0.11 | 0.740 |
| Rivaroxaban, n (%) | 142 (78.02) | 54 (80.60) | 0.19 | 0.663 |
| Aspirin, n (%) | 126 (69.23) | 48 (71.64) | 0.14 | 0.708 |
| Clopidogrel, n (%) | 56 (30.77) | 19 (28.36) | 0.14 | 0.708 |
- Abbreviations: MLAAO, minimal left atrial appendage occlusion; SLAAO, standard left atrial appendage occlusion.
4 DISCUSSIONS
The major findings of the present study are as follows: (1) Both SLAAO and MLAAO demonstrated high procedural success rates and safety. (2) Although the MLAAO group exhibited longer fluoroscopy time, higher radiation exposure dose, and greater contrast medium compared to the SLAAO group, there were significantly reductions in procedure time and hospital stay duration in the MLAAO group. Significant differences were also observed in terms of oropharyngeal/esophageal injury, hypotension, and dysphagia between the SLAAO group and the MLAAO group. (3) The occluder compression ratio measured by fluoroscopy was founded to be lower than that measured by TEE, but a good correlation was observed between the two methods. (4) Both SLAAO and MLAAO procedures demonstrated effectiveness in preventing embolic stroke in patients with NVAF, as evidenced by the 3-month follow-up results.
The SLAAO procedure typically involves general anesthesia, and is guided by CTA, TEE, and fluoroscopy. However, anesthesia-related risks are inherent in the SLAAO, necessitating the simplification of the LAAO procedure for the benefit of patients. The concept of such simplification has been explored for over 20 years, primarily focusing on Amplatzer occluders rather than Watchman occluders.9, 10 Currently, many centers utilize TEE guidance with sedation alone.11, 12 In some centers and cases, LAAO has even been performed without prior TEE or CT.13 TEE provides the advantage of confirming the absence of intracardiac thrombus before the procedure, evaluating the LAA size, shape, and atrial septum characteristics during puncturing, and detecting pericardial effusion. However, TEE is an invasive examination method requiring anesthesia and sedation, and carries potential risks associated with anesthesia and esophageal injury. Moreover, it is contraindicated in certain patients. Intracardiac echocardiography (ICE)-guided LAAO under local anesthesia has been considered a safe and effective alternative to TEE for percutaneous LAAO.9, 14, 15 However, its lower cost-effectiveness restricts its widespread clinical use. Therefore, the introduction of the MLAAO may provide an alternative procedure for a larger number of eligible patients requiring LAAO.
Regarding LAAO, CTA plays a crucial role as a visualized and measured scan. It serves as an important tool for thrombus elimination, demonstrating a weighted mean sensitivity and a negative predictive value of 100%.16 CTA measurements of the ostium diameter and depth of the LAA have been found to be greater than those obtained by TEE and DSA, with CTA showing the strongest relevance and concordance.8, 17 In our study, all patients underwent CTA 24 h before LAAO, with a 60-second delay. 3DR, VR, and MIP techniques were employed to obtain multi-directional anatomical information about the LAA and facilitate the selection of an appropriate occluder size. Our results demonstrated a high degree of consistency between the LAA morphology obtained from MIP and fluoroscopic imaging (Figure 4), underscoring the valuable guidance provided by CTA in LAAO,8, 18, 19 particularly in the context of MLAAO.
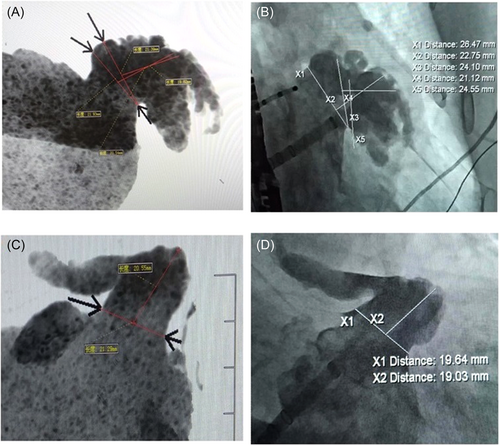
The safety and feasibility of MLAAO have previously been evaluated.4-7 In our study, we found no statistically significant difference in the rate of successful device implantation between the MLAAO group and the SLAAO group. Similarly, there was no significant difference in device resizing, deployment attempts per procedure, device size, incidence of residual flow, and occluder shoulder exposure between the two groups. These findings are consistent with the previous reports.
It is important to note that the MLAAO group exhibited higher radiation exposure dose and contrast medium usage compared to the SLAAO group. This can be attributed to the need for multi-angle angiography to confirm the fluoroscopic “PASS” principle. It should be considered that patients with renal dysfunction may not be suitable candidates for MLAAO due to the increased contrast medium usage. However, we observed significantly shorter procedure times and hospital stay durations in the MLAAO group compared to the SLAAO group. Furthermore, there were significant differences in anesthesia and TEE-related complications, including oropharyngeal/esophageal injury, hypotension, and dysphagia between the two groups. These findings suggest that the MLAAO offers greater cost-effectiveness and safety compared to the SLAAO.
According to the “PASS” principle, the appropriate compression ratio of the Watchman occluder is an important factor in ensuring safety and preventing occluder displacement.20 In SLAAO, the occluder compression ratio is often assessed using TEE. However, in MLAAO, the occluder compression ratio is assessed solely through fluoroscopy. Therefore, it is necessary to establish the consistency and relationship between the occluder compression ratio measured by TEE and fluoroscopy. In our study, we initially evaluated the consistency of the two measurement methods in the SLAAO group. The results revealed that the occluder compression ratio measured with fluoroscopy was lower than that measured with TEE, but there was a good correlation between the two measurements, indicating the reliability of fluoroscopy in assessing the occluder compression ratio. These findings are consistent with the reports by Zhang et al.4
Furthermore, we compared the results of the follow-up 3 months after LAAO between the SLAAO group and the MLAAO group using CTA. CTA serves as a suitable alternative to TEE for post-LAAO device surveillance and is more sensitive than TEE in detecting residual flow.21 Our analysis showed no significant differences in the incidence of device displacement, residual flow, DRT, stroke, or bleeding between the two groups. However, the incidence of residual flow within the range of 1–5 mm was higher in the MLAAO group compared to the SLAAO group. Additionally, one case in the MLAAO group exhibited residual flow exceeding 5 mm. The follow-up results confirmed the safety and effectiveness of MLAAO in preventing embolic stroke in patients with NVAF. However, they also indicated the presence of technical challenges and the need for optimizing MLAAO to achieve greater occlusion.22
4.1 Limitations
This study has several limitations that should be acknowledged. First, it is a single-center retrospective study, which introduces the possibility of selection bias. Second, the sample size of this study is relatively small. Therefore, performing larger-scale study is necessary to validate and confirm the quantified clinical safety and efficacy endpoints, such as the incidence of residual leakage of devices, bleeding complications, and stroke. Finally, a longer follow-up period is needed to assess the long-term safety and effectiveness of MLAAO.
5 CONCLUSION
MLAAO, guided by fluoroscopy without the need for TEE and general anesthesia, appears to be safe, effective, and feasible, particularly for patients who are unable to tolerate or unwilling to undergo TEE and general anesthesia. Furthermore, MLAAO simplifies the procedural steps and facilitates the application of LAAO. However, it is important to note that further validation in a larger number of patients is needed, and there remain technical challenges in the optimization of MLAAO to achieve greater occlusion.
ACKNOWLEDGMENTS
The study was supported by Jiangsu Provincial Medical Innovation Team (Grant No. CXTDB2017015), Jiangsu Commission of Health, China (Grant No. H201665).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.