Iliofemoral artery predilation prior to transfemoral transcatheter aortic valve implantation in patients with aortic valve stenosis and advanced peripheral artery disease
This paper is part of the master and PhD program in medical sciences of the “Universidad Nacional Autónoma de México” (UNAM).
Abstract
Objectives
To investigate the feasibility and safety of percutaneous transluminal angioplasty (PTA) of the iliofemoral arteries (IFA) before transfemoral transcatheter aortic valve implantation (Tf-TAVI) in patients with advanced peripheral artery disease (PAD).
Background
Although Tf-TAVI represents the access of choice, alternative vascular access routes are preferred for patients displaying advanced PAD. PTA of the IFA represents a less invasive option, broadening the spectrum of patients eligible for Tf-TAVI.
Methods
All patients requiring PTA of the IFA before Tf-TAVI, between 2012 and 2021, were included. Primary efficacy endpoint was the rate of successful transcatheter heart valve (THV) delivery and implantation. Primary safety endpoint was the rate of PTA and access-site-related vascular complications, procedural- and in-hospital complications.
Results
Among 2726 Tf-TAVI procedures, 59 patients required IFA predilation. Successful THV delivery and implantation was achieved in 57 (96.6%) patients, respectively. Sheath placement was achieved in 59 (100%) patients with only one minor dissection and no major vascular complications following iliofemoral PTA. Regarding access site complications, two (3.4%) vessel perforations and one (1.7%) vessel rupture were observed, with eight (13.5%) patients requiring unplanned endovascular interventions. There was one intraprocedural death due to THV-induced vessel laceration, while in-hospital all-cause mortality was 8.5% in the present high-risk patient cohort.
Conclusions
Predilation of IFA is safe and effective in patients with advanced PAD. Careful preprocedural planning is paramount in improving procedural safety and efficacy. This strategy has the potential to broaden the spectrum of patients eligible for Tf-TAVI.
Abbreviations
-
- CFA
-
- common femoral artery
-
- CIA
-
- common iliac artery
-
- CT
-
- computed tomography
-
- EIA
-
- external iliac artery
-
- IFA
-
- iliofemoral arteries
-
- MLD
-
- minimal lumen diameter
-
- PAD
-
- peripheral artery disease
-
- PTA
-
- percutaneous transluminal angioplasty
-
- TAVI
-
- transcatheter aortic valve implantation
-
- Tf
-
- transfemoral
-
- VARC-3
-
- valve academic research consortium-3
1 INTRODUCTION
Aortic stenosis (AS) is the most common valvular heart disease in older patients.1 Over the last 10 years, transcatheter aortic valve implantation (TAVI) has become the default therapeutic option for patients with severe symptomatic AS and moderate to high perioperative risk2-5; more recently, noninferiority (and even superiority for selected outcomes) of TAVI over surgical aortic valve replacement (SAVR) was demonstrated in patients at low perioperative risk.6, 7
Although a transfemoral (Tf) approach represents the access of choice in the majority of TAVI procedures,8-10 approximately 25% of patients requiring TAVI display significant peripheral artery disease (PAD).11 Patients with advanced PAD have traditionally been deemed unsuitable for a Tf-TAVI procedure and alternative vascular access routes remained standard of care in such cases. Besides transapical and trans-aortic access, which were consistently linked to increased morbidity and procedural complications,12 a variety of alternative access routes have been evaluated in recent years, including trans-subclavian, trans-carotid or transcaval access, with choice of final vascular access being mainly influenced by centers' expertise and procedural efficacy and safety.10, 12-15
Among patients undergoing Tf-TAVI, presence of significant PAD was shown to increase short- and long-term mortality as well as the risk of vascular complications 16 as compared to patients without significant PAD.17 Patients with PAD may manifest with different degrees of stenosis severity, minimal lumen diameter, circumferential and longitudinal extension of calcification and tortuosity of the iliac vessels, up to combinations that preclude transfemoral delivery of transcatheter heart valves (THV).18 Use of angioplasty of the iliofemoral axes to allow safe sheath and THV delivery represents an appealing and significantly less invasive option, which has the potential to significantly broaden the spectrum of patients eligible for TAVI and to reduce the need for nontransfemoral access routes. Only recently, initial experience with intravascular lithotripsy (IVL) of the iliofemoral axes to facilitate TAVI was reported with promising results.19
Against this background, we aimed to investigate the safety and efficacy of percutaneous transluminal angioplasty (PTA)-facilitated Tf-TAVI in a retrospective, all-comers, single-center cohort.
2 METHODS
2.1 Study population
This retrospective observational analysis evaluated all consecutive patients undergoing Tf-TAVI following multidisciplinary heart team evaluation at the department of cardiovascular diseases of the German Heart Centre, Munich, Germany. A minimalistic approach 20 was used in all cases, while the choice of THV was at the discretion of the operator and influenced mainly by anatomical factors, including aortic annulus size, calcium distribution, and severity as well as coronary height.
Procedural information was captured in a customized database and screened for all patients treated between April 2012 and November 2021. During this period, 2726 Tf-TAVI procedures were performed using commercially available balloon-expandable (n = 2012) and self-expanding (n = 714) THV systems. All patients requiring PTA of the iliofemoral arteries, either upfront or as a bailout strategy (following unsuccessful initial sheath delivery) were included in the present report.
The study was performed in accordance with the principles of the Declaration of Helsinki and all patients provided written informed consent for the procedure. A vascular ultrasound was performed before discharge in all patients to exclude silent vascular injury or underestimated vascular stenoses or residual dissections. Patients were monitored for the occurrence of adverse events during the hospital stay, while clinical follow-up was performed by office visit, phone contact, or structured follow-up letter.
2.2 Computed tomography (CT)
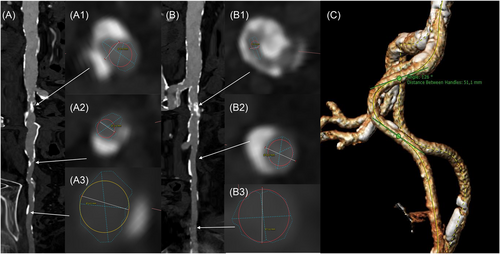
Multislice computed tomography (MSCT) was analyzed by one cardiologist (Hector A. Alvarez-Covarrubias) with extensive experience in the evaluation of TAVI CT. Based on MSCT reconstruction, qualitative and quantitative measures of valve size and calcification as well as degree of peripheral vessel calcification and size were performed by multi-planar reconstruction (MPR) using the 3-Mensio software (Pie Medical Imaging). Aortic and peripheral vessel calcification grading as well as iliac and aortic tortuosity angulation were analyzed according to the endovascular aneurysm repair (EVAR) guidelines.21 Briefly, peripheral vessels were anatomically divided into infrarenal abdominal aorta, common iliac artery (CIA), external iliac artery (EIA), and common femoral artery (CFA); the degree of calcification was graded from 0 to 4 for each segment, according to the percentage of the circumference occupied by calcium (0 = no calcification; 1 = <25%; 2 = 25%–49%; 3 = 50%–75%; 4 = >75%–circumferential); additionally, the minimal lumen diameter (MLD) was measured (Figure 1A,B). Vessel angulation was measured at the most acute angle in the abdominal aorta and the iliac artery segments; tangent angle function was applied between two neighboring vascular segments including the acute angle. Vessel angle was calculated as: 180°—tangent angle (Figure 1C).
2.3 Procedural technique
Vessel access was obtained through a standard percutaneous Seldinger technique in all patients. Subsequently, two suture-mediated closure systems (Perclose ProGlide - Abbott Vascular) were placed using the parallel technique.22 The decision to perform upfront predilation of the iliofemoral arteries was mainly guided by the combination of stenosis severity, circumferential and longitudinal extension of calcification and tortuosity of the iliofemoral vessels on preprocedural MSCT analysis. Such measurements served also as a guidance regarding size of the noncompliant (NC) peripheral balloons being used, with a balloon-to-artery ratio <0.9 in all patients. Prior insertion of the large bore sheath, the NC balloons were advanced through 8–10 French sheath over an extra-stiff wire and inflated up to the lowest pressure necessary to allow full balloon expansion. Following their introduction in the clinical routine, IVL balloons (Shockwave Medical) were used, either as a standalone strategy or in combination with NC balloons, using the maximal number of pulses available. Following peripheral artery predilation, the TAVI sheath was advanced and valve implantation performed as per clinical routine. After valve implantation, the sheath was removed and the suture-based closure systems were used to close the arterial access site, with a safety wire in place to allow for placement of additional plug-based closure devices, if needed. Finally, a digital subtraction angiography was performed to confirm vessel patency and absence of major vascular complications, such as active bleeding, flow-limiting dissections or significant stenoses (Figure 2). In case of persistent relevant bleeding, presence of relevant stenosis or dissection, covered or bare-metal stents were deployed through the contralateral access.

2.4 Endpoints
The primary efficacy endpoint was represented by the rates of successful THV delivery and successful THV implantation. The primary safety endpoint was represented by the rates of PTA-related vascular complications, access-site-related complications, procedural-related complications, and in-hospital complications. Vascular complications were evaluated in accordance with the Valve Academic Research Consortium (VARC)-3 criteria.23
2.5 Statistical analysis
Categorical variables were reported as counts and percentages. Continuous variables were inspected by means of the Shapiro–Wilk test regarding normality of distribution and reported as mean ± standard deviation or median and interquartile range accordingly. Statistical analysis was performed using SPSS Statistics Version 28.0.
3 RESULTS
3.1 Patient population
Between April 2012 and November 2021, 59 patients with severe aortic valve stenosis and advanced PAD underwent iliofemoral artery predilation, either as upfront or as bailout strategy, to facilitate Tf-TAVI. Baseline clinical and echocardiographic characteristics of the patient cohort are shown in Table 1. The patient population displayed a mean age of 80.5 ± 6.3 years, a high prevalence of concomitant coronary artery disease (91.5%), a left ventricular ejection fraction of 60% [48; 60] and a moderate to high perioperative risk in the majority of the patients (EuroScore I: 18.8 [9.6; 32.7]; EuroScore II 6.1 [2.5; 11.6]).
| N = 59 | |
|---|---|
| Age, years | 80.5 ± 6.3 |
| Sex, female | 26 (44.1) |
| Height (cm) | 169.6 ± 8.7 |
| Weight (kg) | 76.4 ± 14.2 |
| Body mass index (kg/m2) | 26.4 ± 3.9 |
| Body surface area (m2) | 1.88 ± 0.20 |
| Arterial hypertension | 58 (98.3) |
| Diabetes mellitus | 25 (42.4) |
| Dyslipidemia | 47 (79.7) |
| Smoking history | 24 (40.7) |
| Atrial fibrillation | 24 (40.7) |
| Chronic obstructive pulmonary disease | 9 (15.3) |
| New York Heart Association Class ≥ III | 34 (57.6) |
| Previous pacemaker | 8 (13.6) |
| Coronary artery disease | 54 (91.5) |
| Previous percutaneous coronary intervention | 29 (49.2) |
| Previous myocardial infarction | 14 (23.7) |
| Previous coronary artery bypass grafting | 16 (27.1) |
| Previous surgical aortic valve replacement | 8 (13.6) |
| History of cancer | 8 (13.6) |
| Serum creatinine (mg/dL) | 1.17 [0.96; 1.41] |
| Estimated glomerular filtration rate (mL/min) | 58 [38; 72] |
| Chronic dialysis | 3 (5.1) |
| Previous stroke/transient ischemic attack | 14 (23.7) |
| Poor mobility | 5 (8.5) |
| Left ventricular ejection fraction (%) | 60 [48; 60] |
| Aortic valve area (cm2) | 0.76 [0.58; 0.88] |
| Mean transvalvular pressure gradient (mmHg) | 39.2 ± 15.4 |
| Maximal transvalvular pressure gradient (mmHg) | 64.7 ± 22.0 |
| Maximal transvalvular flow velocity (cm/s) | 395 ± 73 |
| Systolic pulmonary artery pressure (mmHg) | 42.4 ± 13.3 |
| Hemoglobin (g/dL) | 12.6 ± 1.9 |
| EuroScore I | 18.8 [9.6; 32.7] |
| EuroScore II | 6.1 [2.5; 11.6] |
- Note: Data are shown as counts (%), mean ± SD (standard deviation), or median [25th–75th percentiles].
3.2 CT analysis
CT characteristics are shown in Table 2. Mild aortic tortuosity (aortic angulation 140–159°) was observed in 39 (67.2%) patients, moderate tortuosity (120–139°) in 6 (10.3%), and severe (90-119°) in 1 (1.7%). Calcification involving >75% of the infrarenal abdominal aorta was observed in 53 patients (91.4%). The mean most acute angle was 88 ± 24° on the right and 94 ± 22° on the left iliac arteries. The MLD was 5.3 ± 2.1 mm and 4.9 ± 1.7 mm for the right and left CIA, 4.5 ± 1.4 mm and 4.7 ± 1.6 mm for the right and left EIA and 4.7 ± 1.5 mm and 4.6 ± 1.4 mm for the right and left CFA, respectively. Circumferential vessel calcification (>75°) was observed in 47 (81.1%) and 49 (84.5%) patients regarding the right and left CIA, 23 (39.7%) and 25 (43.1%) patients regarding the right and left EIA, 16 (27.6%) and 16 (27.6%) patients regarding the right and left CFA, respectively.
| N = 58 | |
|---|---|
| Aortic tortuosity (degrees) | |
|
12 (20.7) |
|
39 (67.2) |
|
6 (10.3) |
|
1 (1.7) |
| Infrarenal abdominal aortic calcification | |
|
3 (5.2) |
|
1 (1.7) |
|
1 (1.7) |
|
53 (91.4) |
| Most acute iliac vessel angulation, degrees | |
|
88.28 ± 24.85 |
|
94.07 ± 22.07 |
| More than one iliac angulation less than 90 degrees | |
|
6 (10.3) |
|
8 (13.8) |
| Minimum lumen diameter | |
|
|
|
5.3 ± 2.1 |
|
4.5 ± 1.4 |
|
4.7 ± 1.5 |
|
|
|
4.9 ± 1.7 |
|
4.7 ± 1.6 |
|
4.6 ± 1.4 |
| Circumferential calcification of the peripheral vessels | |
|
|
|
|
|
1 (1.7) |
|
4 (6.9) |
|
6 (10.3) |
|
47 (81.1) |
|
|
|
6 (10.3) |
|
11 (19) |
|
11 (19) |
|
7 (12.1) |
|
23 (39.7) |
|
|
|
3 (5.2) |
|
12 (20.7) |
|
13 (22.4) |
|
14 (24.1) |
|
16 (27.6) |
|
|
|
|
|
1 (1.7) |
|
1 (1.7) |
|
4 (6.9) |
|
3 (5.2) |
|
49 (84.5) |
|
|
|
5 (8.6) |
|
11 (19) |
|
10 (17.2) |
|
7 (12.1) |
|
25 (43.1) |
|
|
|
1 (1.7) |
|
14 (24.1) |
|
14 (24.1) |
|
13 (22.4) |
|
16 (27.6) |
- Note: Data are shown as counts (%), mean ± SD (standard deviation), or median [25th–75th percentiles].
3.3 Procedural characteristics
Procedural characteristics are shown in Table 3. Vessel access was obtained through a percutaneous Seldinger technique in all patients, with no elective surgical vessel exposure being performed. Iliofemoral predilation was performed upfront in 29 (49.2%) and as a bailout strategy in 30 (50.8%) patients. Fifty-three (89.8%) patients were treated with NC balloon only, two (3.4%) patients with IVL only, while in four (6.8%) patients a combined strategy was used (IVL and NC balloon). The most frequently used NC balloon diameters were represented by the 7 mm (16.9%) and 8 mm (66.1%) balloons. A single NC balloon was used in 42 (71.2%) patients, two balloons in 14 (23.7%) patients and more than two balloons were necessary in three (5.1%) patients. The maximal balloon inflation pressure was 14 ± 4.5 atmospheres. Sheath exchange was required in 12 (20.3%) patients, due to initial sheath damage following repeated advancement attempts. A 14 F sheath was used in 49 (83.1%), 16F in 9 (15.3%) cases and 24F in one (1.7%) patient. Balloon-expandable THVs (Sapien XT, Sapien 3 and Sapien 3 Ultra - Edwards LifeSciences) were used in 47 (82.5%) patients, while self-expanding THVs (Acurate Neo 2 and Lotus edge—Boston Scientific; Evolut R - Medtronic; Centera—Edwards LifeSciences) were used in 10 (17.5%) patients (further details are reported in Table 3).
| N = 59 | |
|---|---|
| Elective procedure | 57 (96.6) |
| Oro-tracheal intubation | |
|
4 (6.8) |
|
2 (3.4) |
| Valve-in-valve procedure | 9 (15.3) |
| Procedure time (min) | 66 [51; 83] |
| Fluoroscopy time (min) | 15.8 [11.5; 21.5] |
| Fluoroscopy dose (cGy*cm2) | 1692.8 [622.9; 3428.8] |
| Contrast medium (mL) | 160 [129; 215] |
| Main access | |
|
33 (55.9) |
|
26 (44.1) |
|
3.82 ± 1.17 |
|
0.80 ± 0.24 |
| PTA strategy | |
|
29 (49.2) |
|
30 (50.8) |
| Type of percutaneous transluminal angioplasty | |
|
53 (89.8) |
|
2 (3.4) |
|
4 (6.8) |
| Maximal diameter of noncompliant high-pressure balloon | |
|
1 (1.7) |
|
10 (16.9) |
|
39 (66.1) |
|
9 (15.3) |
| Intravascular lithotripsy balloon diameter | |
|
1 (1.7) |
|
4 (6.8) |
| Number of noncompliant high-pressure balloon used | |
|
42 (71.2) |
|
14 (23.7) |
|
3 (5.1) |
| Maximal balloon inflation pressure, atm | 14 ± 4.5 |
| Number of intravascular lithotripsy pulses applied | 300 [300; 300] |
| Relation between intravascular lithotripsy and noncompliant high-pressure balloon diameter | |
|
1 (1.7) |
|
2 (3.4) |
|
2 (3.4) |
| Sheath type | |
|
50 (84.7) |
|
1 (1.7) |
|
6 (10.2) |
|
1 (1.7) |
|
1 (1.7) |
| Sheath size (Fr) | |
|
49 (83.1) |
|
9 (15.3) |
|
1 (1.7) |
| Need for sheath exchange | 12 (20.3) |
| Transcatheter heart valve implanted | 57/59 |
|
1 (1.8) |
|
24 (42.1) |
|
22 (38.6) |
|
2 (3.5) |
|
5 (8.8) |
|
2 (3.5) |
|
1 (1.8) |
| Aortic valve predilation | 34 (57.6) |
| Aortic valve postdilation | 19 (32.2) |
| Access-site closure | |
|
55 (93.2) |
|
2 (3.4) |
|
1 (1.7) |
|
1 (1.7) |
- Note: Data are shown as counts (%), mean ± SD (standard deviation), or median [25th–75th percentiles].
3.4 Primary efficacy and safety endpoints
Successful THV delivery as well as successful THV implantation were observed in 57 (96.6%) patients (Table 4). THV delivery was unsuccessful in 2 (3.4%) patients; in one case, following successful upfront IVL and NC balloon iliofemoral angioplasty and sheath placement, repeated advancement attempts of a balloon-expandable THV led to partial extroversion of the valve frame, with ensuing vessel laceration, which, despite placement of a stopping balloon in the abdominal aorta and covered stent implantation, led to refractory hemorrhagic shock and intraprocedural death. In the second case, despite successful sheath placement, THV valve advancement proved impossible and, following THV and sheath removal, percutaneous placement of a covered stent was required to achieve hemostasis at the access site; the patient died at the 3rd postprocedural day, due to cardiogenic shock. In-hospital all-cause mortality was 8.5% (five patients); in addition to the aforementioned patients, one (1.7%) patient presented annulus rupture, one (1.7%) presented cardiogenic shock and one (1.7%) had sudden death at the 3rd postprocedural day. Table 5 shows PTA- and access-site-related complications. Importantly, only one minor dissection and no major vascular complications was observed following PTA of the iliofemoral vessels. Regarding access site complications, there were two (3.4%) vessel perforations and one (1.7%) vessel rupture, while eight (13.5%) patients required unplanned endovascular interventions (one [1.7%] balloon dilatation, five [8.5%] covered stent, and two [3.4%] bare metal stent implantation). Only one (1.7%) conversion to surgery was required.
| N = 59 | |
|---|---|
| Successful transcatheter heart valve delivery | 57 (96.6) |
| Successful transcatheter heart valve implantation | 57 (96.6) |
| Multiple valves | 0 (0) |
| In hospital all-cause mortality | 5 (8.5) |
|
2 (3.4) |
|
1 (1.7) |
|
1 (1.7) |
|
1 (1.7) |
| Stroke | |
|
0 (0) |
|
1 (1.7) |
| Acute myocardial infarction | |
|
0 (0) |
|
1 (1.7) |
| New permanent pacemaker | 5 (8.5) |
| Acute kidney injurya | 7 (11.9) |
| Degree of prosthetic aortic valve regurgitation | |
|
|
|
28 (47.5) |
|
28 (47.5) |
|
1 (1.7) |
|
|
|
53 (89.4) |
|
2 (3.4) |
| Mean transprosthetic gradient (mmHg) | 10 [8; 13.6] |
| Maximal transprosthetic gradient (mmHg) | 20 [15; 26.8] |
| Postprocedural systolic pulmonary artery pressure (mmHg) | 38.9 ± 11.1 |
| Postprocedural serum creatinine (mg/mL) | 1.05 [0.87; 1.42] |
| Postprocedural hemoglobin value (gr/dL) | 11.1 ± 1.7 |
| Minimal postprocedural hemoglobin value (gr/dL) | 10.2 ± 1.6 |
| Length of hospital stay (days) | 4 [3; 5] |
| Length of intensive care unit stay (days) | 1 [1; 2] |
- Note: Data are shown as counts (%), mean ± SD (standard deviation), or median [25th–75th percentiles].
- a Acute kidney injury was defined according to RIFLE criteria.
| N = 59 | |
|---|---|
| PTA related complications | |
|
0 (0) |
|
0 (0) |
|
1 (1.7) |
|
0 (0) |
| Access-site related complications | |
|
2 (3.4) |
|
1 (1.7) |
|
5 (8.5) |
|
1 (1.7) |
|
0 (0) |
|
2 (3.4) |
|
2 (3.4) |
|
4 (6.8) |
|
1 (1.7) |
|
0 (0) |
|
0 (0) |
|
2 (3.4) |
|
16 (27.1) |
|
3 (5.1) |
|
8 (13.5) |
|
1 (1.7) |
|
5 (8.5) |
|
2 (3.4) |
| Acute procedural and technical valve-related complications | |
|
1 (1.7) |
|
0 (0) |
|
0 (0) |
|
0 (0) |
| Cardiac structural complications | |
|
1 (1.7) |
|
1 (1.7) |
|
0 (0) |
- Note: Data are shown as counts (%), mean ± SD (standard deviation), or median [25th–75th percentiles]. Abbreviations: BARC, Bleeding Academic Research Consortium classification; PTA, percutaneous transluminal angioplasty.
4 DISCUSSION
This single-centre, retrospective, observational study investigated the efficacy and safety of iliofemoral artery predilation to facilitate Tf-TAVI in patients with advanced PAD. By avoiding the morbidity associated with transthoracic approaches and providing favorable operating room ergonomics, a Tf access represents the access of choice used in the overwhelming majority of TAVI procedures. According to current guidelines, the feasibility of Tf-TAVI access represents one of the dominant features influencing the choice between TAVI and SAVR.24 PAD is a common finding in patients requiring TAVI,11 and alternative access is usually recommended in patients with a MLD of the iliofemoral vessels <5 mm, severe vessel tortuosity and/or calcification, chronic arterial dissection/thrombus, morbid obesity, or severe abdominal aortic atherosclerosis.25
In the present registry, upfront or bailout iliofemoral artery predilation allowed sheath placement in 100% of patients, while THV delivery and successful THV implantation was achieved in 96.6% of this patient cohort, respectively. Considering the pronounced reduction in residual vascular lumen, the marked longitudinal extension and circumferential nature of vessel calcification in the vast majority of patients in addition to the significant vessel tortuosity, the aforementioned results speak for the excellent efficacy of iliofemoral artery predilation in facilitating Tf-TAVI in patients otherwise ineligible for such access. In addition, predilation of iliofemoral arteries displayed an excellent safety profile, with one minor dissection and no major vascular complications following iliofemoral PTA and sheath advancement. However, despite successful vessel predilatation, THV delivery proved impossible in two (3.4%) patients. There was one intraprocedural death due to THV-induced vessel laceration with ensuing refractory hemorrhagic shock and one annulus rupture which required immediate patient transfer for urgent cardiac surgery; in-hospital all-cause mortality ranged at 8.5% in the present patient cohort with a high prevalence of high or prohibitive surgical risk.
Following a recently reported prospective registry evaluating the use of peripheral IVL to facilitate Tf-TAVI,19 the present report represents the second largest data set investigating the feasibility and safety of iliofemoral artery pre-dilation to allow Tf-TAVI in patients with advanced PAD. Due to the retrospective, all-comers nature of the present registry, the vast majority of iliofemoral vessel dilatation was performed by means of noncompliant high-pressure balloons, with IVL being used in 10.2% of patients. IVL is a new technique that uses acoustic shockwaves in a balloon-based system to induce fracture of calcium deposits thereby facilitating luminal gain and vessel compliance.26 Since IVL technology relies on acoustic shockwaves rather than high-pressure inflation to fracture calcified plaques, postulated advantages of such therapy include a reduced risk of barotrauma-induced vessel perforation and/or dissection.26 Importantly, in the present registry, a high-pressure balloon-based strategy in the vast majority of patients allowed successful sheath placement in 100% of patients, with an excellent safety profile. One potential explanation for such a favorable safety profile could be represented by the relative downsizing of balloon diameters used in this patient collective (balloon-to-artery ratio <0.9 in all cases), while IVL applications in the setting of calcified coronary lesions have used a balloon-to-artery ratio of ≈1.27
Closure device failure displayed numerically lower values (3.4% vs. 5.9%) in the present report compared to the aforementioned registry, while need for unplanned endovascular interventions appeared broadly comparable between the two registries (13.5% vs. 12.0%). While the incidence of closure device failure appears nonsignificantly different from that reported in studies including patients without significant PAD undergoing Tf-TAVI,28 need for unplanned endovascular interventions appears more frequent in patients with as opposed to those without significant PAD.28, 29 On the other hand, endovascular repair techniques represent an extremely effective way of managing vascular complications associated with Tf access in patients with advanced PAD.
Since no direct comparisons in terms of efficacy and safety between balloon-based techniques are available, only indirect comparisons, with obvious major inherent limitations, between the patient outcomes reported in such registries may be attempted actually. As long as no clear superiority in terms of efficacy and/or safety of one method upon the other is supported by available data, the lower costs associated with the use of conventional PTA balloons may represent an argument supporting their use instead of alternative techniques at present.
The results of the present report need to be interpreted in the context of the currently available evidence regarding efficacy and safety of alternative TAVI access routes. Use of transapical and transaortic access has considerably declined in recent years since their use has been consistently linked to increased morbidity and procedural complications.12 On the other hand, trans-subclavian, and transcarotid approaches have been increasingly used as alternative vascular access routes, when TF access was deemed prohibitive, depending on centers' expertise. A recent propensity score-based matching analysis from the national prospective FRANCE TAVI registry confirmed the safety of both approaches as compared to the standard Tf access.15 Moreover, the feasibility and clinical outcomes of a transcaval access were evaluated in a prospective observational study including 100 patients, ineligible for TF access and at high or prohibitive risk for transthoracic access.13 Device success, defined as successful transcaval access and deployment of a closure device without death or emergency surgical rescue, was achieved in 98% of the patients. The observed 30-day mortality rate amounted to 8%. Rates of major vascular complications, life-threatening bleeding, and major bleeding were 19.2%, 12.1%, and 6.1%, respectively. Our results display similar efficacy and a more favorable safety profile in terms of major vascular complications compared to the aforementioned results. Additionally, it is important to mention that in patients at advanced age with multiple comorbidities, vessel wall calcifications tend to extend across multiple vascular territories representing significant obstacles to alternative vascular accesses as well; a paradigmatic example of the diffuse nature of vasculopathy is represented by the circumferential nature of infrarenal aortic calcifications in 91.4% of patients in the present registry, which would have represented a considerable hurdle to transcaval access, since identification of calcium-free crossing targets in the abdominal aorta are a prerequisite for the safe performance of this procedure.30
4.1 Study limitations
Some limitations of the present study need to be acknowledged. Despite its moderately large sample size, it is a single center observational study and, due to its retrospective nature, a selection bias cannot be rule out. Additionally, the relatively limited number of patients undergoing IVL-based iliofemoral artery predilation, precludes the possibility of reasonable comparisons between IVL- and NC balloon-based Tf-TAVI outcomes.
5 CONCLUSIONS
Upfront or bailout predilation of iliofemoral arteries is safe and effective in facilitating Tf-TAVI in patients with severe aortic valve stenosis and significant PAD. Careful preprocedural CT analysis, procedural planning, and elaboration of a detailed endovascular bail-out plan for the management of vascular complications are paramount in improving procedural safety and efficacy. This strategy has the potential to significantly broaden the spectrum of patients eligible for Tf-TAVI and to reduce the need for nontransfemoral access routes.
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
Sebastian Kufner reports speaker and consultant fees from AstraZeneca, Bristol Myers Squibb, and Translumina, not related to the current work; the other authors have no conflicts of interest to declare.