Novel technique for transcatheter closure of sinus venosus atrial septal defect: The temporary suture-holding technique
Abstract
Background
Transcatheter repair of sinus venosus atrial septal defect (SVASD) has become an alternative option to surgical repair. There are potential significant complications related to stent stability in the superior vena cava (SVC) and potential migration of the stent that need to be addressed. Therefore, the technique is still evolving.
Objectives
To report results of a new modification “the suture technique” that improves safety profile of positioning and securing a covered stent in the SVC.
Methods
This is a descriptive, single center, retrospective review of patients who underwent SVASD closure using the suture technique at our institution between 02/2020 and 08/2022.
Results
Fourteen patients underwent transcatheter repair of SVASD using the suture technique. All procedures were successful. The suture technique allowed precise stent placement in all patients without any migration or complication. Six patients required additional stent placement at the level of the SVC. One patient had an additional covered stent placed to eliminate a tiny residual shunt. Two patients had negligible residual shunts at the time of the procedure. At follow-up, all patients clinically improved and had significant reduction in right heart size on echocardiography and/or magnetic resonance imaging. No arrhythmia was reported in any patient. None required re-intervention after a mean follow-up of 16.5 ± SD 10.5 months.
Conclusions
The suture technique appears to be safe modification. Although our study involves small sample size with no comparative group, we believe our technique offers greater control over stent positioning, reducing the risk of stent embolization and residual shunting in transcatheter closure of SVASD.
Abbreviations
-
- ASD
-
- atrial septal defect
-
- BMS
-
- bare metal stent
-
- LA
-
- left atrium
-
- LFV
-
- left femoral vein
-
- PA
-
- pulmonary artery
-
- PAPVD
-
- partial anomalous pulmonary venous drainage
-
- RA
-
- right atrium
-
- RFV
-
- right femoral vein
-
- RIJV
-
- right internal jugular vein
-
- RUPV
-
- right upper pulmonary vein
-
- SVASD
-
- sinus venosus ASD
-
- SVC
-
- superior vena cava
-
- TEE
-
- transesophageal echocardiography
1 INTRODUCTION
Until recently, sinus venosus atrial septal defects (SVASD) were exclusively surgically corrected. Over the last 6 years, transcatheter closure has emerged as an alternative to open heart surgery with more institutions reporting their experience.1-15 Different operators used different techniques to implant a covered stent in the superior vena cava (SVC) to close the SVASD and to redirect flow of the anomalous vein back to the left atrium (LA). Since the initial report in Frankfurt at CSI meeting (by Hussein Abdullah, MD and colleagues) and the series from Evelina Children's hospital in London (Hansen),1 strategies have evolved to minimize stent instability and embolization as well as to minimize residual shunting. Multiple techniques have been described to improve the outcome. Some place a bare metal stent (BMS) overlapping the previously placed covered stent to anchor it in the SVC.2 Others prefer to sandwich the covered stents between two BMS stents.3 In this approach, a first stent is placed in the SVC followed by the insertion of the covered stent then a new BMS inserted to sandwich and secure the covered stent. All those techniques aimed to secure the atrial septal defect (ASD) stent in position by trapping it between the wall of the SVC or a stent and an additional stent. In most cases, the securing is performed after covered stent placement thereby not eliminating the risk of embolization. Despite tremendous efforts, stent instability and embolization remain serious problems when dealing with SVASD. In addition, the procedure is a one-shot procedure. After balloon inflation of the covered stent, there is no turning back or adjustment possible. Therefore, precise stent placement is needed to achieve both stability and ASD closure. The foreshortening of about 30%–35% for large diameter stents makes stent positioning extremely challenging to predict. Most of the time, the interventionist may need to compromise between a secured stent in the SVC (that will always be preferred) and complete coverage of the ASD. As a result, residual shunting has been reported with a rate of up to 60%.3 The arrival to the market of longer 10 Zig CP covered stents (NuMED Inc.) have only partially resolved these problems.
In this report, we describe a new simple technique that facilitates SVASD transcatheter closure by securing the covered stents throughout the procedure, allowing for multiple stent adjustments and reduction of residual shunt, stent instability and embolization.
2 METHODS
2.1 Patients
Between February 2020 and August 2022, we identified patients in whom the technique for transcatheter SVASD was modified. This report focuses on those patients who had the so-called “suture technique modification.” The technique is described in detail below.
2.2 Precatheterization assessment
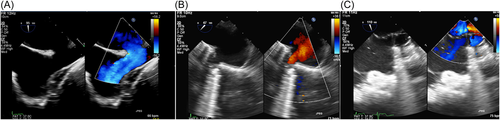
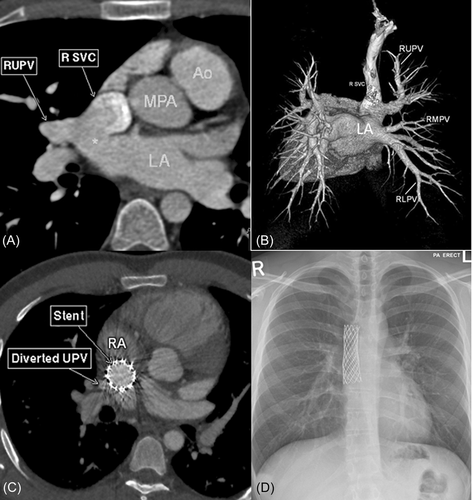
Preprocedure evaluation included 2D/3D echocardiography (Figure 1), cardiac computed tomography (CT) (Figure 2) and/or cardiac magnetic resonance imaging (MRI). Ex vivo simulation of stent implantation on a 3D model was performed with the first two cases only.
2.3 Cardiac catheterization
Written informed consent was obtained from all patients. All procedures were performed under general endotracheal anesthesia with continuous transesophageal echocardiography (TEE) monitoring. Conventional transcatheter closure of SVASD has been described in great details previously.3-5 Briefly, three venous accesses were obtained (right and left femoral veins and the right internal jugular vein [RIJV]). The preclosure catheterization assessment included the following steps: (1) TEE and (2) full hemodynamic data including calculation of Qp/Qs. A veno-venous circuit was created connecting right femoral vein (RFV) with RIJV (0.035″, 260 or 300 cm). Then, a SVC angiogram was performed at baseline and with a balloon inflated in the SVC to determine the diameter of the SVC. Patency and rerouting of anomalous pulmonary veins to the LA were confirmed with venogram and simultaneous pressure monitoring in right upper pulmonary vein (RUPV) and LA with balloon fully inflated in the SVC. The stent placement and positioning differed from techniques previously described. The balloon used to occlude the SVC was a noncompliant balloon. This prevented bulging in the mouth of the anomalous vein that may lead to occlusion of this vein. The LFV catheter was retained to monitor pressure in the RUPV until the end of the procedure. If a pulmonary vein stenosis was found after stent placement a balloon was inflated in the pulmonary vein to relief the compression by the stent.
2.4 Description of the suture technique
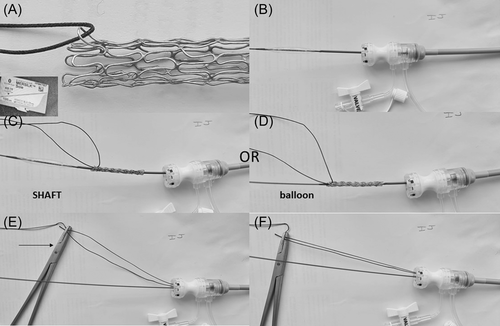
The following technique was developed by the corresponding author (YB) (Figure 3).
For better understanding and to avoid confusion related to the use of proximal and distal ends, extremities of the stents will be described as SVC end for the part of the stent anchoring in the SVC and as right atrial (RA) end for the part closing the ASD. The selection of stent length was based on SVC-ASD length and stent availability. Long stents were used whenever available.
The covered stent was prepared ex vivo as followed: a surgical suture (MERSILK Ethicon KS-75, straight needle, 100-cm long, size 0) was inserted through one of the SVC stent struts. The selected strut was one free of PTFE coverage attachment. The suture was adjusted so that two equal lengths could be held by a needle holder after cutting the needle. The suture itself looped freely around the strut of the stent without a knot. The two extremities of the suture are held by a needle holder after cutting the needle throughout the procedure.
A vascular balloon (12 mm × 6 cm, Mustang; Boston Scientific) was then inserted from the RFV over the wire rail and exteriorized through the RIJV sheath (16 Fr Cook or 14-Fr DrySeal based on the availability). This balloon must be smaller than the SVC diameter and with a shaft long enough to be exteriorized. The chosen covered stent, which was now held by the suture, was crimped over the balloon or its shaft taking care to position the stent with the suture at the SVC side of the stent. Contrary to conventional stent placement where the stent can move from the balloon to the shaft during advancement in the long sheath, here, because the balloon is pulled through the sheath, the stent can slide off the balloon. When crimped on the shaft, a slight balloon inflation facilitates the advancement of the stent–balloon catheter assembly without risk of stent slippage off the balloon. In addition, crimping on the shaft allows to reduce the profile of the stent–balloon assembly by 1–2 Fr (profile of the shaft 5.8 Fr, balloon profile 7 Fr). In practice, we kept the same sheath size but the stent assembly moves more freely.
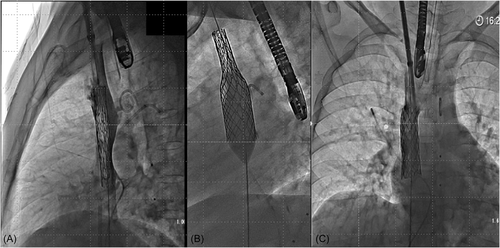
The stent-balloon assembly was advanced from the RIJV to the RA by pushing on the stent and pulling the balloon from the RFV into the RJV sheath. Of note, when a Cook sheath was used, a protective tubing was used to go through the hemostatic valve to avoid damaging the stent cover. The protective sleeve was passed over the balloon onto the shaft with the conical extremity closest to the balloon before mounting the stent and was then retracted to cover the stent. This was not required when DrySeal sheath was used. When needed (when stent was crimped on the shaft), the stent was positioned on the balloon by applying some tension on the suture while pulling on the RFV end of the balloon catheter (Movie S1). Balloon was then inflated to expand the stent in the SVC (Movie S2). While injecting contrast medium from the RIJV sheath, stent-balloon assembly was positioned in the intended final position (Movie S3). At this stage, the stent was free (i.e., not opposed to the wall) in the SVC held by the suture and the stent position can be re-adjusted to have enough stent length in the SVC for good anchoring along with its caudal end below the inferior rim of the defect to cover the ASD (Figure 4 and Movie S4) as confirmed by TEE. The small balloon was then removed (while holding the stent in position with the suture) and replaced by a larger balloon (equal to SVC diameter) with the intention to flare the atrial part of the stent (Movie S5). This step was undertaken under TEE guidance. After deflation of the balloon, the position of the stent was assessed. If too low, some traction was applied to the suture to pull the stent into the SVC. If too high, the balloon was slightly re-inflated in the SVC stent and the stent-balloon assembly was pulled down to have more stent in the RA (Movies S6–S8). When the position was satisfactory, the SVC stent was finally fully inflated to fully oppose the wall and to seal the defect (Figure 5 and Movie S9). To avoid embolization after suture removal, SVC angiography was repeated at this stage to carefully assess if the stent length was long enough in the SVC for adequate anchorage (Movie S10). If the length was deemed inadequate, an overlapping BMS or covered stent was positioned while still holding the covered stent with the suture. Elsewhere, no additional stent needed to be placed (Movie 11). Importantly, the suture was removed by inflating a balloon to hold the stent while pulling on one extremity of the suture until complete removal of the suture. The balloon was then deflated, and wire(s) and catheters were removed. The same technique was used if a BMS was placed to secure the covered stent (Movie S12).
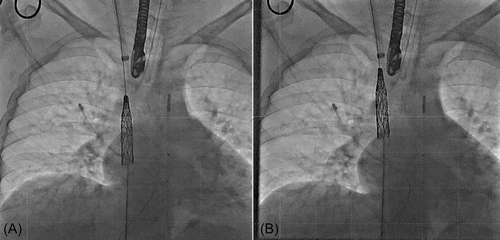
Antibiotic and heparin prophylaxis were given to patients according to institutional protocol. Patients were discharged the following day on 75–100 mg oral aspirin daily. Patient follow-up was performed according to institutional protocol (including clinical examination, electrocardiogram (ECG), and echocardiogram at 1, 6, and 12 months and yearly thereafter. A 24-h ambulatory Holter monitor and a CT/MRI were performed at 6-month follow-up). Adverse events were recorded.
3 RESULTS
The suture technique was performed in 14 patients (mean age: 35.3 ± SD 14.2; range: 5.8–54 years old) during the study period. All patients who underwent this procedure were included in the data capture. The corresponding author (YB) performed 78% of the procedures as single primary operator or with other interventionists. After three cases with some support from the corresponding author, other interventionists were able to perform the procedure on their own without additional support.
Table 1 summarizes patients' baseline characteristics and preprocedural evaluation. All had evidence of right heart overload with Qp:Qs ratio higher than 1.5:1. 10-zig covered CP stents were used in 13/14 cases (the 8 Zig covered CP stent was used in one). The suture technique was successful in all patients allowing secure positioning of the covered stent until final deployment. Two to three repositioning were needed before final deployment. Procedural data are given in Table 2. Six patients needed additional stent at the SVC level to secure the stent (n = 6) and/or to redirect pulmonary vein flow to the LA (n = 2). Three patients had tiny residual shunt at the ASD level. In one case, the decision was made to place an additional covered stent to eliminate the residual shunt. This patient had a small intra-stent clot which resolved after 2 days of heparin infusion. The other two residual shunts were negligible with Qp:Qs ratio close to 1:1. No severe complications occurred during the procedures (see Table 2 for details). No stent embolization was noticed in any case. Median procedural and fluoroscopic times were, respectively, 172 min (range: 110–215) and 27.3 min (range: 15–52). Procedures with longer duration were livestreamed cases during a PICS conference to demonstrate the technique to peers. A total of 12/14 patients were discharged 1 day after the procedure. The patient who had a clot, stayed 3 days for heparin infusion. One patient with hematoma and small pseudo-aneurysm had prolonged compression.
| Case | Age (y) | Gender | Weight (kg) | Diagnosis | Presentation | Preprocedure evaluation |
|---|---|---|---|---|---|---|
| 1 | 5.8 | F | 47 | SVASD, PAPVD (RULPV to SVC-RA junction, RMLPV to RA) | Asymptomatic | CT |
| 2 | 22 | M | 58 | SVASD, PAPVD (RULPV to SVC-RA junction) | Palpitation and SOB for few months | CT |
| 3 | 32 | M | 90 | SVASD, PAPVR (RULPV to right SVC/RA junction and RMLPV to proximal RA) | Asymptomatic | CT and MRI |
| 4 | 39 | F | 68 | SVASD, PAPVD (RULPV to SVC, and RMLPV to SVC-RA junction) | Atypical chest pain and SOB | CT |
| 5 | 43 | M | 69 | PAPVD (RULPV to SVC, RMLPV to SVC-RA and to LA “dual drainage” | Chest pain on exertion for 1 year duration | CT and MRI |
| 6 | 52 | M | 97 | SVASD, PAPVD (RULPV to SVC-RA junction) | Headache, has hypertension | CT |
| 7 | 32 | M | 90 | SVASD, PAPVD (RULPV to SVC, RMLPV to SVC-RA junction) | Chest pain | CT |
| 8 | 36 | M | 74 | SVASD, PAPVD (RULPV to SVC-RA junction) | SOB | CT |
| 9 | 54 | F | 82 | PAPVD (RULPV to SVC-RA junction) | SOB | CT |
| 10 | 13 | M | 42 | PAPVD (RULPV to SVC-RA junction) | murmur | CT |
| 11 | 33 | F | 60 | PAPVD (RULPV to SVC-RA junction) | SOB | CT |
| 12 | 46 | F | 92 | PAPVD (RULPV to SVC-RA junction) | Chest pain | CT |
| 13 | 35 | F | 71 | PAPVD (RULPV to SVC-RA junction) | SOB | CT |
| 14 | 51 | F | 60 | PAPVD (RULPV to SVC-RA junction) | SOB | CT |
- Abbreviations: CT, computed tomography; F, female; LA, left atrium; M, male; MRI, magnetic resonance imaging; PAPVD, partial anomalous pulmonary venous drainage; RA, right atrium; RMLPV, right middle lobe pulmonary vein; RULPV, right upper lobe pulmonary vein; RV, right ventricle; SOB, shortness of breath; SVASD, sinus venosus atrial septal defect; SVC, superior vena cava.
| Case | Qp:Qs | Stent used | Additional stent | Complication | Postprocedure evaluation | Follow-up | Follow-up Echo | Follow-up ECG |
|---|---|---|---|---|---|---|---|---|
| 1 | Pre: 1.5/1, Post: 1/1 | 8-Zig | No | None | No CT | 26 months, asymptomatic | Tiny residual shunt, RV size normalized | Sinus rhythm |
| 2 | Pre: 2 to 1 | 10-Zig 8 cm | No | Small pericardial effusion (no specific treatment) | CT (1 month postprocedure): no residual shunt, patent RUPV | 19 months, asymptomatic | RV size normalized, no residual shunt | Sinus rhythm |
| 3 | Pre: 2.7 to 1 | 10-Zig 8 cm | No | none | CT (Day 3): no residual shunt, RULPV and RMLPV drain to LA with no obstruction | 19 month, asymptomatic | RV size normalized, no residual shunt | Sinus rhythm |
| 4 | Pre: 2 to 1 | 10-Zig 8 cm | Yes, 24 × 48 mm BeGraft to cover residual ASD shunt | Small distal stent clot, not seen on follow-up | CT (the following day to check clot in the stent): Patent stent | 19 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 5 | Pre: 1.8 to 1 | 10-Zig 8 cm | No | None | CT (after 3 months): unobstructed pulmonary veins to LA, significant reduction in RV size | 19 months palpitation, PVC on holter, started on BB | RV size normalized, no residual shunt | Sinus rhythm |
| 6 | Pre: 1.7/1, post: 1.3/1 | 10-Zig 6 cm | No | Small clot, resolved after 2 days Enoxaparin | CT (after 7 months): patent SVC and pulmonary veins, no residual ASD | 14 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 7 | Pre: 2.1/1, post: 1/1 | 10-Zig 8 cm | No | None | CT (after 1 week): patent SVC, no evidence of a residual ASD or excluded pulmonary vein | 19 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 8 | Pre: 1.9/1, post: 1/1 | 10-Zig 6 cm | Yes in the SVC to secure the covered stent (Andra XXL) | None | CT (after 6 months): patent SVC and pulmonary veins, no residual ASD | 14 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 9 | Pre: 1.8/1, post: 1/1 | 10-Zig 6 cm | Yes in the SVC to secure the covered stent (Andra XXL) | None | CT (after 6 months): patent SVC and pulmonary veins, no residual ASD | 14 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 10 | Pre: 2.1/1, post: 1/1 | 10-Zig 6 cm | No | None | CT (after 3 months): patent SVC and pulmonary veins, no residual ASD | 4 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 11 | Pre: 2.2/1, post: 1.1/1 | 10-Zig 7 cm | Yes in the SVC to secure the covered stent and to redirect RUPV (Begraft 24 mm by 37) | None | No CT (scheduled at 6 months postprocedure) | 3.5 months, asymptomatic | No residual shunt | Sinus rhythm |
| 12 | Pre: 2.4/1, post: ND | 10-Zig 6 cm | Yes in the SVC to secure the covered stent (Optimus 57 XXL) | Hematoma | No CT (scheduled at 6 months postprocedure) | 2.5 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 13 | Pre: 2.2/1, post: 1.1/1 | 10-Zig 6 cm | Yes in the SVC to secure the covered stent (Optimus 29 XXL) | None | No CT (scheduled at 6 months postprocedure) | 2 months, asymptomatic | No residual shunt, RV size normalized | Sinus rhythm |
| 14 | Pre: 2.2/1, post: 1.1/1 | 10-Zig 6 cm | Yes in the SVC to secure the covered stent and to redirect RUPV (Begraft 24 mm by 48) | None | No CT (scheduled at 6 months postprocedure) | 1 week | No residual shunt | Sinus rhythm |
- Abbreviations: ASD, atrial septal defect; CT, computed tomography; ECG, electrocardiogram; LA, left atrium; RMLPV, right middle lobe pulmonary vein; RULPV, right upper lobe pulmonary vein; RV, right ventricle; SVC, superior vena cava.
After a median follow-up of 16.5 ± SD 10.5 months (range: 0.5–31.5), no patient had reintervention. When performed, cardiac CT angiography confirmed patency of stents and flow of the redirected pulmonary veins. Patients with short follow-up did not have CT yet. At most recent follow-up, transthoracic echocardiography showed all patients with complete resolution of right-sided volume overload; 12 patients with no residual atrial shunt and 2 patients with tiny residual atrial shunt which were considered hemodynamically insignificant. No late stent migration was noted during the follow-up (Table 2). No arrhythmias or AV block were found on ECG or the Holter recordings.
4 DISCUSSION
Transcatheter closure of SVASD and PAPVD was first described by Hussein Abdullah and colleagues at the Frankfurt CSI meeting in 2013. Since then, several reports have been published from different centers around the world.1-6 The procedure has good immediate, short and midterm outcomes. Hansen reported 1.4 years median follow-up in 25 adult patients who underwent transcatheter closure of SVASD. SVC stent well positioned with patent pulmonary venous flow was demonstrated by TTE and CT.1 MRI studies showed significant reduction in right ventricle dimensions in all patients. Nonetheless, technical difficulties resulting in complications are not uncommon. The difficulty resides in the precise positioning of the covered stent. To hold in position, the stent needs to anchor in the SVC. At the same time, the RA part needs to be long enough, when flared-up, to completely close the ASD. Foreshortening of the stent, especially at large diameter, complicates the positioning of the stent leading to; stent instability and embolization if the SVC portion is too short and to residual shunt if the ASD coverage is inadequate. In the same report by Hansen et al.,1 out of 25 patients, 13 (52%) needed additional stents, 9 to secure the covered stent in SVC (with one embolization) and 4 to close the residual shunting at atrial level. Minimal residual shunting was observed in 15 cases (60%).
The suture holding technique described here helps securing the stent “at SVC side” while flaring up the RA end of the stent. Holding the suture at RIJV sheath, the operator has the advantage of securing and adjusting the stent position during balloon inflation. If the stent is flared too high in the SVC not completely covering the ASD or, on the contrary, too low in the RA, the stent could be repositioned with the help of a balloon and the suture. If inadequate flaring of the stent leads to inadvertent residual shunting with part of the stent crossing the atrial septum, pushing the stent down would not be possible. In this situation, inflating a balloon in the ASD using the wire that is maintained in the RUPV until the end of the procedure, would crimp the stent enough to allow its repositioning in the RA. At any stage, balloon inflation was applied to the wall, at the SVC part of the stent, to additionally secure the stent.
The technique per se does not guarantee the absence of embolization as a case of stent embolization using the suture technique was described in a recent series from another group.3 It is important to note that the stability is warranted until the release of the suture. The risk of embolization is null as the suture is physically holding the stent. As a result, a proper assessment of stent stability is crucial before releasing the suture. Interestingly, the suture gives time to the interventionist to safely assess the need for an additional stent before taking any decision. With techniques described so far, the assessment is done, in the contrary, without any certainty of the stability.
Like in any techniques, after covered stent placement, the decision-making to add an additional stent is done by assessing the amount of stent present in the SVC. With the suture technique, if this assessment is done accurately, then, the risk of embolization is minimized. The embolization reported by another group happened after the removal of the suture. For optimal stability, the stent assembly should extend at least to the level of the azygos vein. If the SVC part of the stent is too short, a BMS needs to be placed. It is only when once the desired position of the stent is achieved, that the suture can be released. In this series, an additional stent was needed to secure the covered stent in six patients. In all of them, long CP stents were not available and the need for an additional stent was obvious from the beginning of the procedure. It is extremely important to note that to avoid pulling up the covered stent while removing the suture, a balloon should be inflated within the stent. The same technique has to be applied if an additional BMS is placed. Furthermore, it should be noted that as a result of entrapment of the suture between the SVC wall and the balloon or the SVC wall, the BMS and the balloon some tension has to be applied on the suture to release it.
4.1 Study limitation and future directions
This article describes a small sample size case series. Long-term follow-up is missing. However, the technique is extremely easy to implement. After 2–3 cases, this technique was used by other interventionists in the institution without the help of the corresponding author. This technique can be used at any age as it allows to reduce the size of the sheath needed for the procedure (stent balloon assembly: 12 mm balloon+stent compared to 18–28 mm balloon+stent). The first patient included in the series was 5.8 years old showing that the technique can be extended to this population. In our opinion, the technique is easier in children as SVC diameter is usually smaller than in adults. We presently offer this approach whenever the SVC is larger or equal to 16-mm in diameter as at this diameter no further intervention will be needed. However, we acknowledge that the transcatheter SVASD closure is still controversial in this age group even within the interventionists' community. Long-term studies will be needed to confirm our statement.
The suture can alternatively be placed from the femoral vein and exteriorized in the jugular vein as reported by Haddad and colleagues.16 Using a BIB balloon to deliver the covered stent had the advantage of using a single balloon but has a large profile requiring larger sheaths and limits the positioning and adjustment once the inner balloon is inflated.
5 CONCLUSION
Although it is a small cohort with no comparative group, we believe the suture technique is a novel and safe modification of conventional SVASD exclusion using a covered stent. This technique allows precise positioning of the covered stent with multiple adjustments. In addition, the stent is secured until the removal of the suture giving time to the operator to decide if an additional stent in the SVC is required to stabilize the covered stent.
CONFLICTS OF INTEREST
ZMH is a consultant for NuMED Inc., manufacturer of the CP stent. Other authors have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.