A prospective trial of a novel low-dose paclitaxel-coated balloon therapy in patients with restenosis in drug-eluting coronary stents Intracoronary Stenting and Angiographic Results: Optimizing Treatment of Drug Eluting Stent In-stent REstenosis 3A (ISAR-DESIRE 3A)
Funding information: Boston Scientific Corporation, Grant/Award Number: Partial funding
Abstract
Objectives
We investigated the clinical efficacy of a paclitaxel-coated balloon (PCB) with a novel matrix coating and reduced drug concentration in comparison with a widely used PCB with iopromide excipient.
Methods
We prospectively enrolled patients with restenosis in drug-eluting stents. All patients were treated with a novel low-dose PCB with citrate-based excipient (Agent PCB). Angiographic follow-up was scheduled at 6–8 months. Outcomes were compared against those of patients treated with iopromide excipient PCB (SeQuent Please PCB) enrolled in a trial with identical inclusion and exclusion criteria. The primary endpoint was percent diameter stenosis (%DS) at follow-up angiography. The primary hypothesis was that the investigational device would be non-inferior to the control device (ClinicalTrials.gov Identifier: NCT02367495).
Results
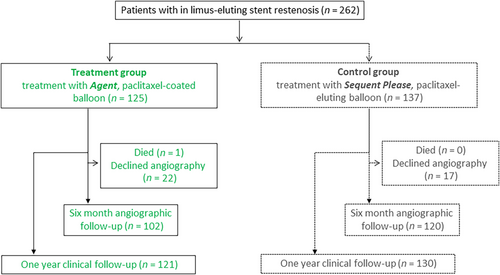
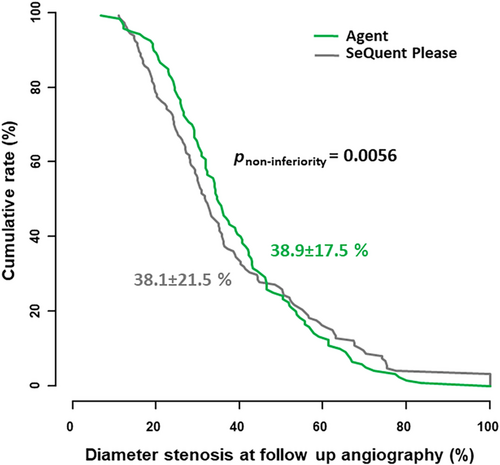
One hundred twenty-five patients with 151 lesions were enrolled. Mean age was 68.1 ± 10.2 years, 40.8% had diabetes mellitus and 80.1% had focal morphology in-stent restenosis. Follow-up angiography data at 6–8 months was available for 102 (81.6%) patients. The Agent PCB was non-inferior to the SeQuent Please PCB in terms of the primary endpoint (38.9 ± 17.5 vs. 38.1 ± 21.5%; p non-inferiority = 0.0056). Late lumen loss was also comparable between the groups (0.35 ± 0.55 vs. 0.37 ± 0.59; p = 0.71). There was no difference between the groups in the incidence of TLR (27.7% vs. 22.1%; p = 0.31), death or myocardial infarction (4.2% vs. 4.4%; p = 0.92) or target lesion thrombosis (1.0% vs. 0.7%; p = 0.93).
Conclusion
In patients with DES restenosis, angioplasty with a novel PCB with citrate-based excipient was non-inferior to PCB with iopromide excipient in terms of angiographic outcome.
1 INTRODUCTION
Although management of patients presenting with coronary restenosis has improved significantly over the last decades, recurrence rates after treatment remain substantial.1 Drug-coated balloon (DCB) angioplasty represents an effective treatment approach and avoids implantation of additional stent layers.2 Due to the challenges associated with drug delivery and transfer, all approved devices with randomized clinical trial evidence have used paclitaxel as the active agent. Recently, however, some concerns have emerged regarding long-term safety after paclitaxel-based catheter treatment for peripheral arterial disease and some analyses suggested a dose-dependent adverse effect.3 Although analyses of clinical trials for coronary use did not show evidence of an adverse safety signal,4 refinements of DCB catheter technologies incorporating lower drug concentration may represent a preferable approach.
The Agent paclitaxel balloon catheter (paclitaxel-coated balloon [PCB]; Boston Scientific, Natick, MA, USA) catheter uses a novel citrate based excipient and a lower drug dosage (2.0 mcg/mm2) compared with conventional PCB catheters. In order to assess its performance in clinical practice, we conducted the prospective, historical control Intracoronary Stenting and Angiographic Results: Drug Eluting Stent In-Stent Restenosis 3A (ISAR-DESIRE 3A) trial. Using an investigative protocol analogous to the ISAR-DESIRE 3 trial,5 we enrolled patients with clinically significant restenosis undergoing treatment with the citrate-based excipient PCB. We aimed to compare angiographic and clinical outcomes against the benchmark PCB with iopromide excipient (SeQuent Please PCB, B. Braun Melsungen AG, Melsungen, Germany).5-9
2 MATERIALS AND METHODS
2.1 Study design and population
The ISAR-DESIRE 3A trial is a prospective, two-center, non-randomized, single arm trial with a historical control-group. Eligible patients were enrolled at two centers—Deutsches Herzzentrum München, Technische Universität München, Munich and Krankenhaus Landshut-Achdorf, Medizinische Klinik I, Landshut, both in Germany—and treated with a novel PCB with citrate-based excipient and a lower drug dosage (Agent PCB; Boston Scientific, Natick, MA, USA). The historical control group comprised patients assigned to treatment with conventional PCB with iopromide excipient (SeQuent Please) in the setting of the randomized ISAR DESIRE 3 trial.5
The inclusion and exclusion criteria were identical in both ISAR-DESIRE 3A and ISAR-DESIRE 3. Eligible patients were at least 18 years of age with ischemic symptoms or signs of myocardial ischemia and a restenosis ≥ 50% diameter stenosis (DS) after prior implantation of a drug-eluting stent in a native coronary vessel. Written, informed consent signed by the patient was required for participation and a negative pregnancy test was mandatory in women with childbearing potential. Exclusion criteria were cardiogenic shock, acute ST-elevation myocardial infarction within 48 h from symptom onset, target lesion located in the left main trunk, a bypass graft or in a vessel sized < 2.0 mm, malignancies or other comorbid conditions with life expectancy less than 12 months or that may result in protocol non-compliance, severe renal insufficiency (glomerular filtration rate ≤ 30 ml/min) and contraindications to antiplatelet therapy or paclitaxel. The study was conducted according to the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices. The trial protocol and informed consent was approved by the institutional ethics committee responsible for the participating centers.
2.2 Study devices, procedure, and follow-up
The Agent PCB coating matrix consists of a stable citrate-ester based excipient incorporating a reduced dose of paclitaxel (2 μg paclitaxel per mm2 of the balloon). Patients in the historical control group had been assigned to treatment with a conventional PCB with iopromide excipient (SeQuent Please PCB; paclitaxel dose: 3 μg per mm2 of the balloon).
Patients who met all of the inclusion criteria and none of the exclusion criteria were enrolled. Study inclusion was performed in the catheterization laboratory after wiring of the target lesion. Time zero was defined as the time of inclusion. Patients were considered enrolled in the study and eligible for the final intention-to-treat analysis at the time of inclusion. After enrollment, the target lesion was dilated with standard balloon angioplasty followed by angioplasty with a PCB. The assigned treatment approach had to be used for all restenotic lesions in those patients who required interventional treatment in multiple restenotic lesions and the use of more than one paclitaxel-eluting balloon per lesion was allowed. Indications for bail-out stenting were large dissections with flow limitation and residual stenosis >50% after multiple balloon dilations.
Immediately after the decision to perform the intervention, patients received 500 mg aspirin intravenously (if they did not receive it within the prior 12 h) and body-weight adjusted intra-arterial or intravenous heparin. After receiving a loading dose before the intervention, all patients received afterwards aspirin indefinitely and a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) at daily maintenance doses using standard local practice for at least 6 months. Cardiac medications were prescribed according to the discretion of the patient's physician.
ECG recordings were performed immediately after intervention and then daily until discharge. Blood samples for the determination of cardiac markers (CK, CK-MB, troponin, and high-sensitivity troponin where available) were drawn on the day after intervention and thereafter daily until discharge and for all suspected ischemic events. Telephone or in-hospital clinical follow-up was performed after 1 and 12 months. All patients were scheduled for routine angiographic surveillance after 6–8 months.
2.3 Data management
Relevant data were collected and entered into a computer database by personnel of the Clinical Data Management Centre (ISAResearch Center, Munich. Germany). All events were adjudicated and classified by an independent event adjudication committee. Baseline, post procedural, and follow-up coronary angiograms were digitally recorded and assessed off-line in the quantitative coronary angiographic (QCA) core laboratory (ISAResearch Center, Munich, Germany) with an automated edge-detection system (QAngio XA version 7.1, Medis Medical Imaging Systems, Leiden, Netherlands) by operators unaware of the treatment allocation. Measurements were performed on cineangiograms recorded after the intracoronary administration of nitroglycerine. Baseline QCA measurements were performed using the single worst view projection for the index lesion; the same view projection was used for the measurements after intervention. In the follow-up angiogram, the QCA measurements were performed using the single worst-view projection at that time point. The contrast-filled non-tapered catheter tip was used for calibration. In the follow-up angiogram, the in-segment area was defined as the treated area as well as 5 mm margins proximal and distal to the treated area. Restenosis morphology was adjudicated according to criteria modified from Mehran et al.10
2.4 Endpoints and definition
The primary endpoint was in-segment percent DS at 6–8 month follow-up angiography. Secondary angiography endpoints were assessed at 6–8 month follow-up angiography and included in-segment minimal lumen diameter; in-segment binary angiographic restenosis, defined as DS ≥50% in the in-segment area (including the interventional and the proximal and distal 5 mm margins); and late lumen loss in the treated area, defined as the difference between minimal lumen diameter post-procedure and minimal lumen diameter at follow-up angiography.
Secondary clinical endpoints were assessed at the 12 months follow-up and included the incidence of target lesion revascularization (TLR), defined as any revascularization procedure involving the target lesion due to luminal renarrowing in the presence of symptoms or objective signs of ischemia; the composite of death or myocardial infarction; and the incidence of target lesion thrombosis modified from the Academic Research Consortium criteria.11 The definition of myocardial infarction has been described previously,12 which considered clinical symptoms, cardiac biomarkers, and ECG.
2.5 Sample size calculation and statistical analysis
The primary objective of the study was to assess the non-inferiority of the PCB with citrate-based excipient compared with the results of the PCB with iopromide excipient in the setting of the ISAR-DESIRE 3 trial.5 The null hypothesis was that PCB with citrate-based excipient was inferior to the PCB with iopromide excipient in terms of the primary outcome of percent DS at 6–8 months follow-up angiography. Sample size calculation for the non-inferiority analysis was based on following assumptions: percent DS of 35% after both paclitaxel-eluting balloons, non-inferiority margin (∆) of 7% absolute (20% of assumed percent DS of 35%), one-sided α-level of 0.05 and power of 80%. We estimated that 102 patients were needed to be enrolled. To account for possible losses to follow-up, it was planned to enroll 125 patients.
Categorical variables are presented as counts and percentages and compared by chi-squared or Fisher's exact test, whereas continuous variables are displayed as mean with standard deviation and compared by Student's t-test. The Kaplan–Meier method was uses to calculate cumulative event rates and comparison was made by using a Cox proportional hazards model. The non-inferiority hypothesis was tested with EquivTest (Statistical Solutions, Cork, Ireland) according to the methods described by Hauck and Anderson. Non-inferiority was considered to be demonstrated if the upper 1-sided 95% confidence interval (CI) surrounding the difference between the test treatment and the standard treatment was lower than the prespecified threshold. After the determination of non-inferiority, we performed standard superiority testing with a two-tailed p value 0.05 considered statistically significant. All analyses were performed according to the intention-to-treat principle. Prespecified subsets of interest were old and young patients (above/under the median age), men and women, diabetic and nondiabetic patients, small and large vessels. Sample size calculation was performed with nQuery Advisor (Statistical Solutions, Cork. Ireland). Statistical analysis was performed by using the R 3.5.1 Statistical Package (R Foundation for Statistical Computing, Vienna, Austria).
2.6 Study organization
ISAR-DESIRE 3A is an investigator-initiated trial sponsored by Deutsches Herzzentrum München. The study was funded in part by a research grant to the institution from Boston Scientific. The authors are solely responsible for the design and conduct of the study, all study analyses, the drafting and editing of the manuscript, and its final contents.
3 RESULTS
3.1 Baseline patient, lesion, and procedural characteristics
A total of 125 patients were enrolled and treated with the PCB with citrate-based excipient. The historical control group consisted of the PCB with iopromide excipient within the ISAR-DESIRE 3 trial included 137 patients (Figure 1). The baseline patient characteristics of both treatment groups are displayed in Table 1. No statistically significant differences were found between the two groups.
| Agent | SeQuent Please | ||
|---|---|---|---|
| Paclitaxel coated balloon with citrate ester excipient | Paclitaxel coated balloon with iopromide excipient | ||
| Patients | n = 125 | n = 137 | p value |
| Age | 68.3 ± 10.2 | 67.7 ± 10.5 | 0.69 |
| Female | 30 (24.0) | 32 (23.4) | 0.90 |
| Diabetes mellitus | 51 (40.8) | 56 (40.9) | >0.99 |
| Insulin-dependent | 26 (20.8) | 21 (15.3) | 0.25 |
| Hypertension | 90 (72.0) | 105 (76.6) | 0.39 |
| Hyperlipidaemia | 102 (81.6) | 108 (78.8) | 0.57 |
| Current smoker | 23 (18.4) | 19 (13.9) | 0.32 |
| Prior myocardial infarction | 50 (40.0) | 53 (38.7) | 0.83 |
| Prior bypass surgery | 18 (14.4) | 15 (10.9) | 0.40 |
| Multivessel disease | 112 (89.6) | 129 (94.2) | 0.17 |
| Clinical presentation | |||
| Acute coronary syndrome | 22 (17.6) | 26 (19.0) | 0.77 |
| Ejection fractiona | 54.8 ± 9.6 | 53.6 ± 9.9 | 0.53 |
- Note: Data shown as mean ± SD or number (percentage).
- a Data available for 54.2% of study sample (142 patients).
A total of 151 lesions were treated in the Agent PCB group and 172 lesions were treated in the SeQuent Please PCB group. Angiographic and procedural characteristics at baseline are displayed in Table 2. Lesions in the Agent PCB treatment group had larger reference vessel diameter (2.98 ± 0.50 vs. 2.75 ± 0.50 mm; p < 0.001) and minimal lumen diameter (1.15 ± 0.42 vs. 0.98 ± 0.49 mm; p < 0.001) compared with lesions treated with the SeQuent Please PCB. Predilation was performed more often in lesions treated with the Agent PCB as compared to lesions treated with the SeQuent Please PCB (97.4% vs. 80.8%; p < 0.001). Predilation with a cutting balloon was performed more often in lesions treated with the Agent PCB as compared to lesions treated with the SeQuent Please PCB (6.6% vs. 1.2%; p = 0.01). DS after PCB angioplasty was greater in lesions treated with the Agent PCB as compared to lesions treated with the SeQuent Please PCB (23.9 ± 8.0 vs. 18.5 ± 8.4%, p < 0.001).
| Agent | SeQuent Please | ||
|---|---|---|---|
| Paclitaxel-eluting balloon with citrate ester excipient | Paclitaxel-eluting balloon with iopromide excipient | ||
| Lesions | n = 151 | n = 172 | p value |
| Target vessel | 0.92 | ||
| Left anterior descending | 52 (34.4) | 59 (34.3) | |
| Left circumflex | 46 (30.5) | 54 (31.4) | |
| Right coronary artery | 52 (34.4) | 59 (34.3) | |
| Left main | 0 (0.0) | 0 (0.0) | |
| Bypass graft | 1 (0.7) | 0 (0.0) | |
| Restenosis morphology | 0.12 | ||
| Focal | 109 (72.2) | 101 (58.7) | |
| Multifocal | 12 (7.9) | 18 (10.5) | |
| Diffuse | 27 (17.9) | 44 (25.6) | |
| Proliferative | 1 (0.7) | 3 (1.7) | |
| Occlusive | 2 (1.3) | 6 (3.5) | |
| Bifurcation | 43 (28.9) | 47 (27.3) | 0.76 |
| Ostial | 41 (27.5) | 55 (32.0) | 0.38 |
| Vessel size (mm) | 2.98 ± 0.50 | 2.75 ± 0.50 | <0.001 |
| Lesion length (mm) | 10.1 ± 5.7 | 10.1 ± 6.4 | 0.99 |
| Diameter stenosis, pre (%) | 61.5 ± 12.5 | 64.4 ± 16.9 | 0.09 |
| Minimal lumen diameter, pre (mm) | 1.15 ± 0.42 | 0.98 ± 0.49 | <0.001 |
| Procedures | |||
| Treated as per protocol | 147 (97.4) | 161 (93.6) | 0.11 |
| Predilation | 147 (97.4) | 139 (80.8) | <0.001 |
| Cutting/scoring balloon predilation | 10 (6.6) | 2 (1.2) | 0.01 |
| Balloon pressure, max (atm) | 13.4 ± 3.6 | 13.7 ± 4.1 | 0.56 |
| Minimal lumen diameter, post (mm) | 2.32 ± 0.41 | 2.30 ± 0.45 | 0.67 |
| Diameter stenosis, post (%) | 23.9 ± 8.0 | 18.5 ± 8.4 | <0.001 |
- Note: Data shown as mean ± SD or number (percentage) based on in-stent analysis.
- Abbreviations: NA, not applicable; PEB, paclitaxel-eluting balloon; PES, paclitaxel-eluting stent.
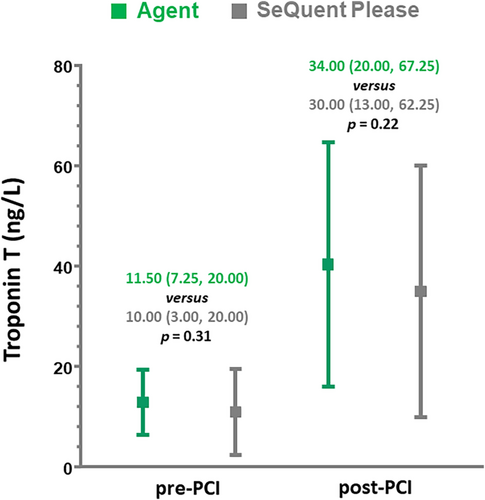
Troponin T (TnT) levels at pre and post-percutaneous coronary intervention (PCI) were found to be comparable between Agent PCB and the SeQuent Please PCB (11.50 [7.25, 20.00] vs. 10.00 [3.00, 20.00] ng/L, p = 0.31 and 34.00 [20.00, 67.25] vs. 30.00 [13.00, 62.25] ng/L, p = 0.22 respectively) (Figure 2).
3.2 Angiographic follow-up
Overall, angiographic follow-up data were available for 222 of 262 patients (84.7%), with no significant differences across the treatment groups (p = 0.18). Results of the angiographic follow-up at 6–8 months are displayed in Table 3. Concerning the primary endpoint- in-segment percent DS, the Agent PCB was found to be non-inferior to the SeQuent Please PCB (38.9% ± 17.5% vs. 38.1% ± 21.5%; p non-inferiority = 0.0056) (Figure 3). Multivariate analysis identified vessel size and DS pre, but not PCB treatment group as independent predictors for the primary outcome.
| Agent | SeQuent Please | ||
|---|---|---|---|
| Paclitaxel-eluting balloon with citrate ester excipient | Paclitaxel-eluting balloon with iopromide excipient | ||
| Lesions | n = 120 | n = 147 | p value |
| Diameter stenosis (%) | 38.9 ± 17.5 | 38.1 ± 21.5 | pnon-inferiority 0.0056 |
| Minimal luminal diameter, (mm) | 1.88 ± 0.64 | 1.79 ± 0.74 | 0.28 |
| Recurrent binary restenosis | 30 (25.0) | 39 (26.5) | 0.78 |
| Late lumen loss (mm) | 0.35 ± 0.55 | 0.37 ± 0.59 | 0.71 |
- Note: Data shown as mean ± SD or number (percentage) based on in-segment analysis.
In the prespecified subgroup analysis, no interaction was observed between the treatment group and the primary endpoint for subgroups defined by age (p-interaction = 0.32), gender (p-interaction = 0.45), diabetic status (p-interaction = 0.40), and vessel size (p-interaction = 0.79).
The Agent PCB and the SeQuent Please PCB showed comparable rates of in-segment binary angiographic restenosis at follow-up (25.0% vs. 26.5%; p = 0.78). Late lumen loss at follow-up was also comparable in both groups (0.35 ± 0.55 vs. 0.37 ± 0.59 mm; p = 0.71).
3.3 Clinical follow-up
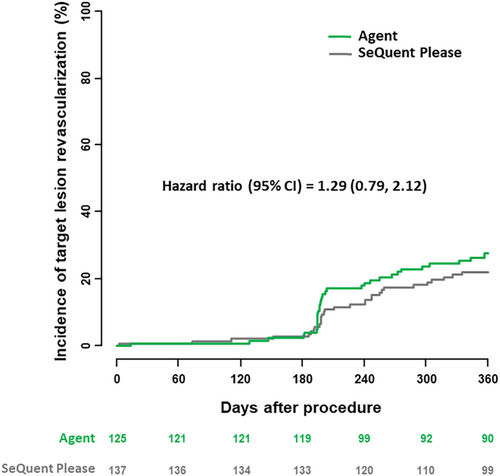
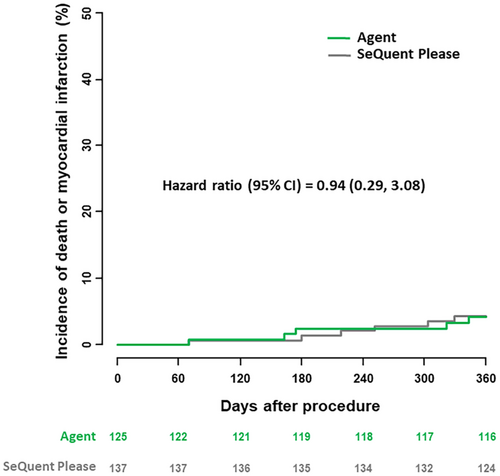
Follow-up to 1 year was complete in 121 of 125 patients in the Agent PCB treatment group and 130 of 137 patients in the SeQuent Please PCB group; in patients with incomplete follow-up mean duration of follow-up were 70 days and 309 days respectively. The clinical results at 1 year are summarized in Table 4. Overall, no statistically relevant differences were observed between the groups. At 1 year, TLR rates were comparable in both treatment groups (27.7% vs. 22.1%, p = 0.31) (Figure 4). Concerning clinical safety at 1 year, adverse event rates were low and comparable in both groups. Rates of the composite of death or myocardial infarction were 4.2% in the Agent PCB group as compared to 4.4% in the SeQuent Please PCB group (p = 0.92) (Figure 5). One target lesion thrombosis occurred in each group (1.0% vs. 0.7%; p = 0.93).
| Agent | SeQuent Please | ||
|---|---|---|---|
| Paclitaxel-eluting balloon with citrate ester excipient | Paclitaxel-eluting balloon with iopromide excipient | ||
| Patients | n = 125 | n = 137 | p value |
| Death | 1 (0.8) | 3 (2.2) | 0.38 |
| Myocardial infarction | 4 (3.4) | 3 (2.2) | 0.58 |
| Target lesion revascularization | 33 (27.7) | 30 (22.1) | 0.31 |
| Coronary artery bypass grafting | 3 (2.5) | 3 (2.2) | 0.90 |
| Percutaneous coronary intervention | 33 (28.5) | 28 (20.6) | 0.20 |
| Death or myocardial infarction | 5 (4.2) | 6 (4.4) | 0.92 |
| Death, myocardial infarction, target lesion revascularization | 37 (31.4) | 32 (23.5) | 0.18 |
| Target lesion thrombosis | 1 (1.0) | 1 (0.7) | 0.9 |
- * Data shown as number (percentages are Kaplan–Meier estimates).
4 DISCUSSION
This study represents the largest study comparing the novel citrate based excipient PCB with lower drug dosing (Agent PCB, Boston Scientific) versus a conventional PCB with iopromide excipient (SeQuent Please PCB, B. Braun) in patients presenting with restenosis within a DES.
The main findings are: (i) the Agent PCB was non-inferior to conventional SeQuent Please PCB in terms of the primary angiographic outcome at 6–8 months follow-up; (ii) both the Agent PCB and the conventional SeQuent Please PCB showed comparable clinical efficacy and safety out to 12 months.
The efficacy of angioplasty with DCB relies on rapid initial transfer from the balloon surface to the vessel wall and retention of the anti-proliferative agent, which is necessary for persistent suppression of cellular proliferation.2 DCB technology is comprised of three key elements: balloon catheter, antiproliferative drug and a spacer or excipient substance. To date the majority of DCB technologies are based on the use of paclitaxel as the active agent. This is because the high lipophilicity—in comparison with sirolimus and its analoges—provides advantages in terms of drug transfer and tissue retention13 Excipient substances facilitate drug transfer and uptake2 and in experimental studies devices using excipient coating showed greater inhibition of neointimal growth compared to devices without excipient.14 Although, a number of different excipients have been employed on clinically approved DCBs, the earliest technology and the most widely studied device to date uses iopromide as excipient—an angiographic contrast medium capable of dissolving paclitaxel at much higher concentrations than saline. Iopromide-based intracoronary local drug delivery was first described in the trials of Scheller et al. suggesting that higher paclitaxel tissue concentrations are achievable with a mixture of iopromide and paclitaxel than with paclitaxel alone.15, 16
Although DCB angioplasty has proven an effective therapy for both coronary in-stent restenosis4 and de novo disease,17 some concerns have emerged regarding late safety particularly in patients undergoing DCB angioplasty for peripheral arterial disease.3 Although analysis of datasets from trials of coronary use have generally failed to detect an adverse safety signal,4 the relatively high dose of paclitaxel per unit used on DCB catheters and the microparticulate debris generated by DCB angioplasty in animal models represent potential targets for device iteration.2
Newer generation DCB technologies have leveraged-modified coating technologies using proprietary coating techniques, alternative excipient substances, and more recently, sirolimus analoges. Broadly speaking the technological goal of novel coatings is to maintain the drug delivery and retention achieved with iopromide-based coatings, while reducing potential adverse effects of DCB dilation, including signs of local vessel wall toxicity and microparticulate embolization from the balloon coating, the latter of which has been implicated as a potential cause of adverse clinical events after angioplasty.18, 19 The Agent PCB incorporates design modifications aimed to increase the integrity of the matrix coating, to reduce microparticulate embolization. The matrix coating consists of a citrate-based excipient with a reduced dose of paclitaxel as compared to the SeQuent Please PCB (2 vs. 3 μg/mm2).
The results of our trial suggest that the Agent PCB with citrate excipient was non-inferior to the benchmark SeQuent Please PCB with iopromide excipient in terms of angiographic antirestenotic efficacy. Both devices demonstrated comparable efficacy in terms of the primary endpoint of percent DS as well as late lumen loss and binary angiographic restenosis. These results are highly suggestive of a broadly comparable clinical performance of the two devices. The results should be interpreted in light of the non-randomized study design and the use of a historical control group for comparison. However, the risks inherent in non-randomized comparisons are mitigated to an extent by the use of a prospectively defined protocol with trial registration, a common treatment protocol in both trials, identical inclusion and exclusion criteria, and the same core laboratory for angiographic analysis.
Although baseline patient characteristics were generally well balanced between the groups, despite the commonalities in study conduct and inclusion and exclusion criteria between the two trials, some lesion and procedural variables were different between the groups. The difference in minimal luminal diameter prior to the procedure was trivial and not likely to be clinically significant. Moreover, although there was a slight difference in mean reference vessel diameter between the groups, the primary endpoint of interest was diameter stenosis, a variable, which is independent of vessel size. On the other hand, the impact of a higher rate of pre dilation (97.4% vs. 80.8%) and a slightly more frequent use of cutting or scoring balloon (6.6% vs. 1.2%) in the Agent PCB group should be considered while interpreting the results. Finally, there was some difference in mean percent DS- post between the groups, however, we chose percent DS at follow-up as the primary endpoint, which does not have a direct contribution from post procedure stenosis in the way that for example late luminal loss does.
Second, in terms of lesion preparation, predilation with both non-compliant balloon and cutting/scoring balloon was more frequently observed in the Agent PCB group. Optimal lesion preparation before PCB is considered to be crucial to enhance drug delivery.20 In the ISAR DESIRE 4, systematic lesion preparation with scoring balloon before PCB resulted in improved antirestenotic efficacy.21 Taking this into account, the non-inferior results of the Agent PCB despite lower paclitaxel dosage may also be related to more frequent lesion preparation with cutting/scoring balloon angioplasty. On the other hand, there were relatively low rates of cutting/scoring balloon use in both groups and any influence on overall angiographic outcome could be expected to be marginal. The lack of influence of differential cutting/scoring balloon use is also supported by the multivariate analysis.
Our results are in keeping with the data reported from the recently published randomized trial of Hamm et al. comparing the same two technologies.22 In that trial, the primary endpoint of late loss was similar in both treatment groups: 0.397 ± 0.43 mm with Agent versus 0.393 ± 0.536 mm with Sequent Please (difference 0.004 mm [95% CI–0.189, 0.196], prespecified non-inferiority margin 0.20 mm, pnon-inferiority 0.046).
We also examined for changes in cardiac biomarker levels post-intervention between treatment groups, on the basis that such a difference, if present, might point to differences in downstream microparticulate embolization from the balloon surface. However, no such differences were evident. This is in keeping with a prior report that showed no difference in biomarker rise in patients with in-stent restenosis randomly allocated to treatment with PCB, repeat stenting or plain balloon angioplasty,23 and speaks against a meaningful impact of embolization of microparticulate matter from PCB at a clinical level in the coronary space.
Concerning clinical efficacy outcomes at 12 months, Agent PCB showed comparable event rates as compared to SeQuent Please PCB. Although, it might be observed that the TLR event rates were numerically higher in the Agent PCB group, this difference was not statistically different and the influence of protocol mandatory angiographic follow-up at 6–8 months should be considered.24 Moreover, although TLR rates beyond 1 year have been reported to be low with SeQuent Please PCB,13 these observations may not be generalizable to the Agent PCB. Larger studies with longer follow-up are required to better characterize the comparative efficacy of both devices. Finally, concerning clinical safety outcomes, event rates were low and comparable in both groups. These results are in line with event rates reported in recent PCB trials, confirming the high-safety profile of balloon-based treatment strategies currently in use.4, 25
4.1 Limitations
In addition to limitations associated with the non-randomized nature of the comparison between the treatment groups as discussed already, a number of other limitations should be borne in mind when interpreting the results. First, selected patients were included in the trial and patients with restenosis within DES implanted in the left main stem or presenting as ST-elevation MI were systematically excluded from the trial. Second, analysis of the primary endpoint is based on incomplete observations (84.7% in the present study). However, this issue is an inherent feature of larger studies with angiographic follow-up and prior analyses support the reliability of such data where angiographic follow-up exceeds 80%.26 Third, the study was not powered to show a difference in clinical endpoints and as such, the lack of a difference in clinical outcomes observed between the two groups does not rule out a difference.
5 CONCLUSIONS
In patients undergoing percutaneous intervention for DES restenosis, angioplasty with a novel PCB with citrate-based excipient was non-inferior to angioplasty with a conventional PCB with iopromide excipient in terms of angiographic antirestenotic efficacy. Both devices showed comparable clinical efficacy and safety out to 12 months, though the trial was not powered to detect differences in this respect.
ACKNOWLEDGMENT
Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
RAB reports research funding to the institution of prior employment from Celonova Biosciences. The other authors report no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.