Final five-year results of the REMEDEE Registry: Real-world experience with the dual-therapy COMBO stent
All authors takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding information: Orbus Neich BV; OrbusNeich Medical B.V.; OrbusNeich, AstraZeneca, and Tryton; The Medicines Company; Biotronik, Biosensors, and Medtronic; Abbott Vascular and Boson Scientific
Abstract
Objectives
This final report from the REMEDEE Registry assessed the long-term safety and efficacy of the dual-therapy COMBO stent in a large unselected patient population.
Background
The bio-engineered COMBO stent (OrbusNeich Medical BV, The Netherlands) is a dual-therapy pro-healing stent. Data of long-term safety and efficacy of the this stent is lacking.
Methods
The prospective, multicenter, investigator-initiated REMEDEE Registry evaluated clinical outcomes after COMBO stent implantation in daily clinical practice. One thousand patients were enrolled between June 2013 and March 2014.
Results
Five-year follow-up data were obtained in 97.2% of patients. At 5-years, target lesion failure (TLF) (composite of cardiac death, target-vessel myocardial infarction, or target lesion revascularization) was present in 145 patients (14.8%). Definite or probable stent thrombosis (ST) occurred in 0.9%, with no additional case beyond 3-years of follow-up. In males, 5-year TLF-rate was 15.6 versus 12.6% in females (p = .22). Patients without diabetes mellitus (DM) had TLF-rate of 11.4%, noninsulin-treated DM 22.7% (p = .001) and insulin-treated DM 41.2% (p < .001). Patients presenting with non-ST segment elevation acute coronary syndrome (NSTE-ACS) had higher incidence of TLF compared to non-ACS (20.4 vs. 13.3%; p = .008), while incidence with STE-ACS was comparable to non-ACS (10.7 vs. 13.3%; p = .43).
Conclusion
Percutaneous coronary intervention with the dual-therapy COMBO stent in unselected patient population shows low rates of TLF and ST to 5 years. Remarkably, no case of ST was noted beyond 3 years.
Abbreviations
-
- ACS
-
- acute coronary syndrome
-
- DAPT
-
- dual antiplatelet therapy
-
- DES
-
- drug-eluting stents
-
- DM
-
- diabetes mellitus
-
- EPCs
-
- endothelial progenitor cells
-
- ITDM
-
- insulin-treated diabetes mellitus
-
- NSTE-ACS
-
- non-ST segment elevation acute coronary syndrome
-
- ST
-
- stent thrombosis
-
- STE-ACS
-
- ST-segment elevation acute coronary syndrome
-
- TLF
-
- target lesion failure
-
- TLR
-
- target lesion revascularization
-
- TV-MI
-
- target-vessel myocardial infarction
1 INTRODUCTION
After the early experience with first-generation drug-eluting stents (DES), long-term follow-up in clinical stent trials became unequivocally important. Although first-generation DES significantly reduced in-stent restenosis by its cytostatic or cytotoxic drug,1, 2 long-term follow-up revealed accrual of late adverse events, in particular stent thrombosis (ST).3 These antiproliferative drugs concomitantly impede endothelial regeneration.4 A delayed healing response may lead to increased incidence of ST, impaired vasomotor response, and delayed restenosis at long term follow-up.5, 6 Therefore, facilitating and accelerating endothelial healing after stent implantation is desirable. Re-endothelialization is a multistep process in which circulating endothelial progenitor cells (EPCs) play an important role.7, 8 EPCs are immature cells that are capable of differentiating into healthy endothelial cells. The dual-therapy COMBO stent (OrbusNeich Medical BV, The Netherlands) is a DES with a biodegradable polymer and actively captures EPCs with its anti-CD34+ antibody layer in order to accelerate endothelial healing; which could also improve long-term outcomes.9 We have previously reported on outcomes up to 4 years with this stent from the REMEDEE registry.10-13 In this final report from the REMEDEE registry, we aim to assess long-term safety and performance of the dual-therapy COMBO stent at 5-year follow-up in a large unselected patient population.
2 METHODS
2.1 Study design
The study design, endpoint definitions, and primary results have previously been described in detail.10 In brief, the REMEDEE Registry is a prospective, multicenter, international, investigator-initiated registry evaluating the safety, and performance of the COMBO stent in daily clinical practice. The procedure and antiplatelet therapy were at investigators' discretion and according to the European Society of Cardiology guidelines. Early cessation of dual antiplatelet therapy (DAPT) (<180 days) occurred in 7.8% and had no influence on occurrence of target lesion failure (TLF) of ST.14 Nine centers throughout Europe participated in this registry. Data were collected at baseline, at the time of intervention, postprocedure, and from follow-up visits at 30 days, 180 days, and yearly up to 5-years. The registry was conducted according to the Declaration of Helsinki and approved by the regional ethical review board affiliated with each participating center. All patients provided informed consent. The study is registered at clinicaltrials.gov (NCT01874002).
2.2 Analysis
The main purpose of the current report was to evaluate the efficacy and safety of the COMBO stent throughout 5-year follow-up. The primary endpoint was TLF. Furthermore, we performed post hoc analysis for the 5-year clinical outcomes according to sex, diabetes status, and clinical presentation.
2.3 Study endpoints and definitions
The primary focus of the current analysis is TLF, defined as a composite of cardiac death, target-vessel myocardial infarction (TV-MI) or any target lesion revascularization (TLR) at 5-year follow-up. MI was defined according to the Third Universal Myocardial Infarction definition,15 other outcomes were defined according to the Academic Research Consortium definitions.16 An independent clinical events committee adjudicated all clinical outcomes.
Diabetes mellitus (DM) was defined as a prior established diagnosis of DM or the use of medication to control blood glucose. Patients using insulin therapy were considered insulin-treated DM (ITDM), patient taking oral medication and/or dietary measures only were considered as non-insulin treated DM patients (non-ITDM). Patients undergoing emergency percutaneous coronary intervention (PCI) for ST-segment elevation MI or PCI for stabilized STEMI were considered as STE-acute coronary syndrome (ACS) patients. Patients undergoing urgent PCI or after stabilizing of non-ST-segment elevation MI or unstable angina were considered as NSTE-ACS patients.17 All other indications for PCI (stable angina, documented ischemia, angiography, other) were categorized as non-ACS.
2.4 Statistical analysis
Data are shown as counts with valid percentages for categorical variables or mean ± SD for continuous variables, unless mentioned otherwise. Lesion characteristics and dimensions were visually estimated by the operator at each site. The incidence of all clinical endpoints was estimated using Kaplan–Meier methods. Follow-up was censored at the last known date of follow-up, or at 60 months, whichever came first. All endpoints were evaluated in the intention-to-treat population. Cox proportional hazard models were used to calculate the hazard ratios within the subgroups and were adjusted for predefined baseline characteristics. A p-value of <.05 was considered statistically significant. All statistical analyses were performed with the SPSS software, version 26.0 (IBM SPSS Statistics, IBM Chicago, IL).
3 RESULTS
3.1 Baseline characteristics
One thousand patients, in whom treatment with a COMBO stent in the setting of routine clinical practice was attempted, were enrolled between June 2013 and March 2014. The baseline, lesion, and procedural characteristics have been described previously10 and are shown in Tables S1 and S2. In summary, the mean age was 65 ± 11 years, 73.9% were male, and 18.4% had DM. Indication for PCI was ACS in 49.8%. A total of 1,255 lesions were treated with a median length of 15.0 mm12-20 and a median reference vessel diameter of 3.0 mm (3.0–3.5). Lesions were classified as American College of Cardiology/American Heart Association type B2 or C in 58.9%. Implantation of at least one study device was achieved in 99.4% of patients.
3.2 Clinical outcomes
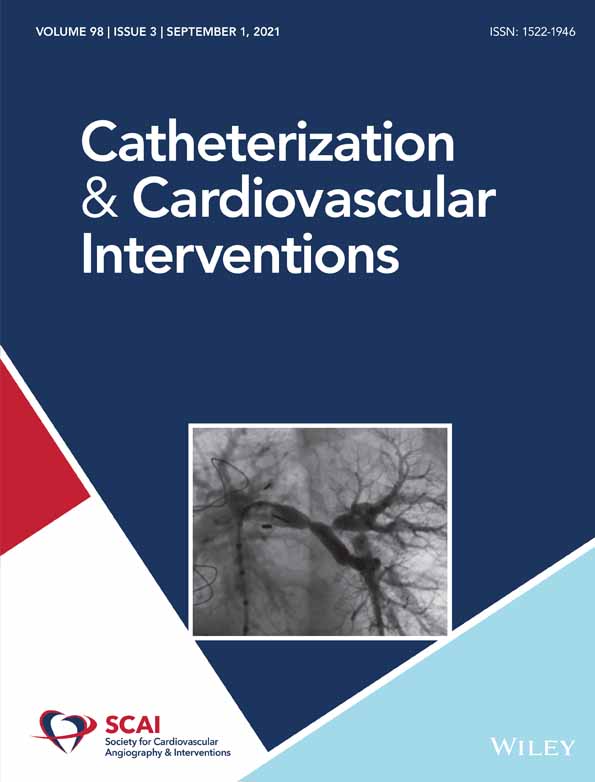
Five-year follow-up was obtained in 97.2%. At 5-year follow-up, TLF occurred in 145 patients (14.8%) with the following incidence of individual component endpoints: cardiac death 6.0%, TV-MI 3.4%, and TLR 8.8% (Table 1, Figure 1). All-cause death was 10.2%, of which 4.6% died a noncardiac death, mostly due to cancer (58.2%) or infection (27.9%). Definite or probable ST occurred in 0.9% at 5-year follow-up. Definite ST occurred in 0.8%. No case of additional ST was noted after 3 years. Descriptive characteristics of the definite ST cases are presented in Table S3.
| Target lesion failurea | 145 | 14.8% |
| All-cause death | 101 | 10.2% |
| Non-cardiac death | 43 | 4.6% |
| Cardiac death | 58 | 6.0% |
| Any myocardial infarction | 57 | 6.0% |
| Target-vessel MI | 32 | 3.4% |
| Target vessel revascularization | 111 | 11.6% |
| Target lesion revascularization | 85 | 8.8% |
| Definite stent thrombosis | 8 | 0.8% |
| Early | 5 | |
| Late | 0 | |
| Very late | 3 | |
| Probable stent thrombosis | 1 | 0.1% |
- Note: Values are n (Kaplan–Meier estimates).
- Abbreviations: MI, myocardial infarction; TLR, target lesion revascularization.
- a Defined as cardiac death, target-vessel MI, TLR.
3.3 Subgroup analysis
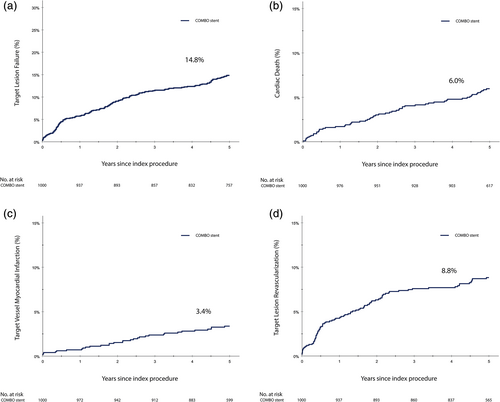
The performance of the COMBO stent did not differ between the sexes (Figure 2). Also after correcting for baseline dissimilarities, no statistically significant sex-differences in 5-year clinical outcomes were found (Table S4). Males treated with COMBO stent had TLF-rate of 15.6% at 5-year compared to 12.6% in females (p = .22), adjusted hazard ratio 0.80; 95% CI (0.52–1.24), (p = .32).
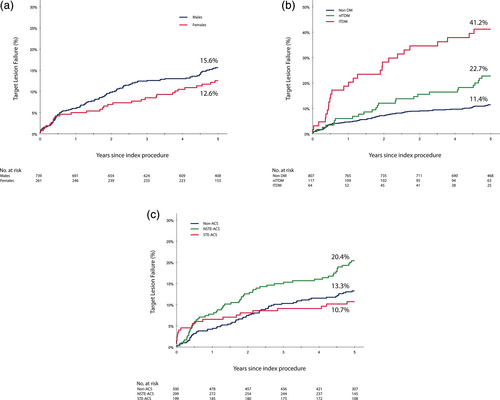
Diabetic patients show a higher risk of TLF compared to non-diabetic patients, especially those who require insulin treatment (Figure 2). Non-ITDM patients had an adjusted 1.78 (95% CI 1.10–2.89) fold higher risk on 5-year TLF compared to non-diabetics. Whereas ITDM patients had an adjusted 3.98 (95% CI 2.47–6.42) fold higher risk of TLF compared to nondiabetic patients. All endpoints according to diabetic status and their adjusted hazard ratios are presented in Table S5.
At 5-year follow-up, the TLF rate was significantly higher in NSTE-ACS patients compared to non-ACS patients (20.4 vs. 13.3%; p = .008) (Figure 2). This difference was mainly driven by the higher rate of TV-MI (10.3 vs. 1.7%; p = .001) (Table S6). The efficacy of the COMBO stent did not differ between STE-ACS and non-ACS patients (TLF 10.7 vs. 13.3%, respectively, p = .43), but STE-ACS suffered from a higher rate of definite/probable ST (2.5 vs. 0.2%; p = .003) (Table S6).
4 DISCUSSION
4.1 Key findings
This study is the first to report 5-year clinical outcomes of the dual-therapy COMBO stent in a large unselected patient population. The main findings are (a) The primary endpoint of TLF occurred in 14.8% at 5-years, (b) definite/probable ST occurred in 0.9% with no case of additional ST noted beyond 3 years, (c) on subgroup analysis, no sex-differences in 5-year TLF were found, whereas the incidence of 5-year TLF was significantly higher among diabetic patients and in patients presenting with NSTE-ACS.
4.2 Long-term efficacy of dual-therapy COMBO stent
Long-term follow-up is essential to determine the true safety and efficacy of coronary artery devices beyond early procedural outcomes.18 Although comparison between different studies must be done with caution—reported events rates are affected by a range of factors other than the stent—we try to interpret our results by comparing to other studies. The incidence of cardiac death and TLR in our registry were similar to those reported at 5 years in the BIOSCIENCE,19 RESOLUTE,20 or TWENTE21 trials. However, the incidence of TV-MI is lower to those reported in BIOSCIENCE, RESOLUTE, or TWENTE trials.19-21 The latter could possibly be explained by difference in endpoint definition. In addition, the REMEDEE Registry did not routinely test cardiac biomarkers post-PCI; therefor non-clinically relevant periprocedural MI's were not reported.
4.3 Long-term safety profile of dual-therapy COMBO stent
Early-generation DES demonstrated an increased risk of late and very late ST compared to bare-metal stents.18 New-generation DES have improved anti-proliferative drug release kinetics with more biocompatible or biodegradable polymer coating and thinner stent struts. However, although low, the annual risk of very late ST continues; several reports indicate a 0.1% rate of very late ST per year.22 The BIOSCIENCE trial demonstrated an incidence of 0.8% very late ST in biodegradable-polymer sirolimus-eluting stents and 1.3% in durable-polymer everolimus-eluting stents.19 Especially beyond 3 years the curves of ST accrue.19 Also, the TWENTE trial shows that the risk of ST continues beyond 1 year and ST occurred throughout 5-years of follow-up.23 In the current registry, we see no ST cases beyond 3 years. Moreover, we found that despite the unselected patient population, the overall incidence of definite ST was encouragingly low and only 0.8% at 5-years. The majority of ST cases occurred acute in STE-ACS patients. A propensity-matched analysis showed that the 2-year rate of definite ST did not differ between COMBO stent and Promus or Resolute stent.24 These findings support the hypothesis behind the dual-therapy technology of low very late ST risk due to functional endothelialization.25 Failure to uphold a functional endothelial barrier within the stented segment has been hypothesized as a mechanism for the development of neoatherosclerosis.26 And neoatherosclerosis is often observed in patients with very late ST.27
In addition, an optical coherence tomography (OCT) serial follow-up study demonstrated that the COMBO stent was associated with an increase in strut coverage from 77% at 2 months to 95% at 5 months.28 In addition, a regression of neointimal hyperplasia between 9 and 24 months was observed.28 The Japan-United States of America Harmonized Assessment by Randomized, Multicenter study of OrbusNeich's Combo Stent (HARMONEE) trial randomized 572 patients to either COMBO or an everolimus-eluting stent.29 The COMBO stent demonstrated superior strut coverage by OCT (91.3 vs. 74.8%, p < .001).29 Both observations may reflect improved vessel healing. However, large clinical randomized trials with long-term follow-up should confirm this low rate of ST during long-term follow-up.
4.4 Subgroups
Estrogen is associated with improved function and recruitment of EPCs from the bone-marrow.30 But a study of Topel et al.31 showed that women with and without coronary artery disease, regardless of menopause status, have lower circulating EPC levels compared to men. Whether this leads to different percentage of endogenous arterial repair response and re-endothelialization cannot be known based on this study design. We found no statistically significant differences between females and males in clinical outcomes despite the baseline differences. Also Chandrasekhar et al.32 showed no statistically significant sex-based differences in clinical outcomes after COMBO stent implantation at 1 year in the combined dataset of the current registry with the MASCOT all comers international registry.
In vitro studies showed that low EPC count or impaired EPC function is a strong predictor of in-stent restenosis, progression of atherosclerosis, and cardiovascular events.33-35 EPC function is impaired with age,36 smoking,37 as well as in patients with type II DM,38 dyslipidemia,39 and hypertension.40 Diabetic patients are also found to be older, have more frequently dyslipidemia and hypertension.41 The impaired EPC function in diabetic patients can be one of the reasons that the COMBO stent does not seem to improve clinical outcomes in diabetic patients and show higher rates of TLR compared to non-diabetics like other DES.42
ACS patients remain a challenging subgroup, at an increased risk of adverse events. Stent studies continue to show higher adverse event rates in ACS.43, 44 Higher rates of cardiac death, MI, and ST often occur early in STE-ACS patients and late complications are more often seen in patients presenting with NSTE-ACS.45 The current report shows similar results. Patients treated with COMBO stent in context of STE-ACS as well as NSTE-ACS had higher risk of TV-MI compared to non-ACS patients. In STE-ACS patients the adverse events occurred mostly within the first 6 months, while in NSTE-ACS patients adverse events occurred mostly beyond 6 months. Although ischemic conditions signal differentiation of CD34+ cells toward EPC phenotypes in order to promote re-endothelialization,25 the use of anti-CD34+ antibodies on the COMBO stent does not prevent the higher risk of TV-MI in ACS patients. Long term follow–up of patients with ACS treated with COMBO stent shows, consistent results with the 1 year findings reported previously,46 with worse outcomes, compared to non-ACS patients. The results of these post hoc analysis should be interpreted with caution. The numerical lower rate of ischemic events in STE-ACS compared with non-ACS is probably caused by the small groups.
5 LIMITATIONS
This is the first large cohort to report long-term results of the COMBO stent in an unselected patient population. All events were monitored and adjudicated by an independent clinical event committee. However, this study is limited by its observational single-arm design and no direct comparison to other DES can be made. In addition, the sub-group analysis is subject to small sample size and possible confounding.
6 CONCLUSIONS
This is the first study to report five-year follow-up of the dual-therapy COMBO stent in a large cohort. The final clinical outcomes of the REMEDEE Registry show low rates of TLF and ST. Remarkably, no case of ST was noted beyond 3 years. More data are needed to confirm this late benefit of the dual-therapy COMBO stent.
ACKNOWLEDGMENTS
The authors greatly acknowledge all the patients for their participation. In addition, the authors are grateful to Dr. Eefting and Dr. Rensing for their work in adjudicating all clinical events. Moreover, the authors especially acknowledge Margriet Klees for her continued efforts regarding the REMEDEE Registry.
CONFLICT OF INTEREST
All including centers received institutional grants. Dr. Erglis has received grant support and personal fee from Abbott Vascular and Boson Scientific; and consultant fee from Biotronik, Biosensors, and Medtronic. Dr. van 't Hof has received grant support from The Medicines Company and grant support from Abbott. Dr. de Winter has received grant support from OrbusNeich, AstraZeneca, and Tryton; and consultant fee from OrbusNeich. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
FUNDING INFORMATION
The Amsterdam UMC, location Academic Medical Center, University of Amsterdam received an unrestricted research grant from OrbusNeich Medical B.V., The Netherlands.
Open Research
DATA AVAILABILITY STATEMENT
Data is available at request.