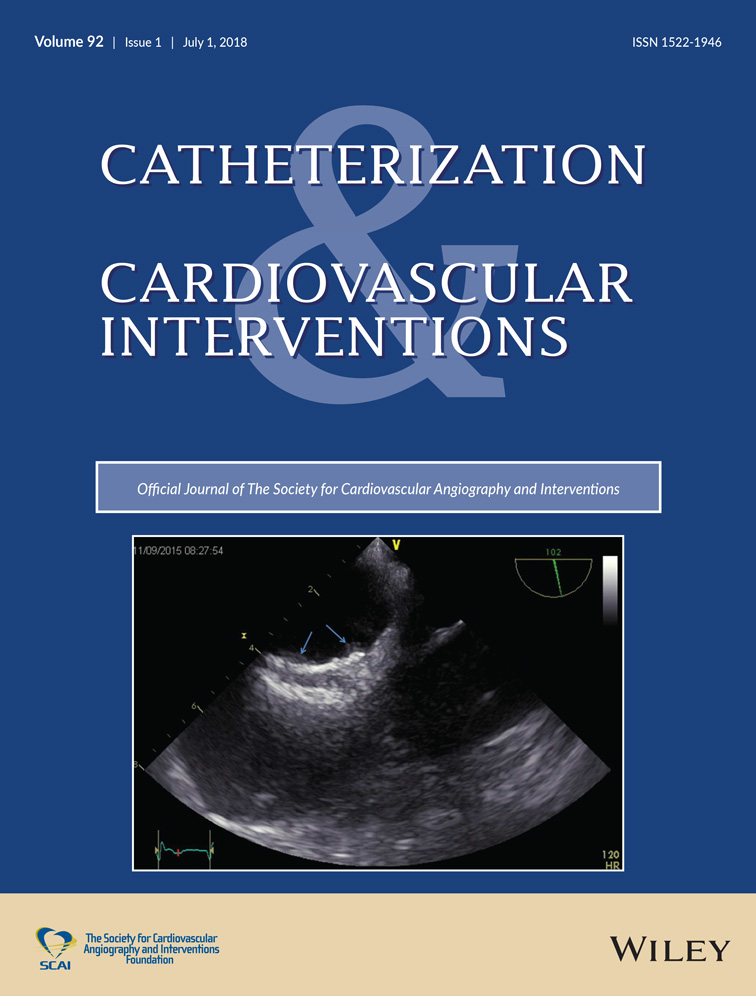
Thromboembolism after WATCHMANTM in a clopidogrel non-responder: A case for concern?
Abstract
Atrial fibrillation (AF) is associated with an increased risk of stroke and thromboembolism (TE). The WATCHMANTM left atrial appendage (LAA) closure device is indicated to reduce the risk of TE from the LAA in patients with non-valvular AF. Here, we present a case of a patient with device-related thrombus who suffered a TE event two months after WATCHMANTM LAA closure and two weeks after switching from aspirin plus warfarin to aspirin plus clopidogrel therapy. Laboratory investigation identified the patient to be hypercoagulable and to be a non-responder to clopidogrel therapy. We discuss the potential role of platelet function testing to prevent device-related thrombi.
CONFLICT OF INTEREST
Dr. Paul Gurbel discloses the following relationships: Grants from Haemonetics, DCRI, Medicure, Merck, NIH, Bayer, Amgen, Medimmune, and Coramed, and personal fees from AstraZenaeca, Boehringer, Merck, Janssen, Bayer, Medicure, and Haemonetics, UptoDate; in addition, Dr. Gurbel has a patent Platelet function testing issued.