Contrast-induced nephropathy
Abstract
Contrast induced nephropathy (CIN) is an iatrogenic disorder, resulting from exposure to contrast media. Contrast-induced hemodynamic and direct cytotoxic effects on renal structures are highly evident in its pathogenesis, whereas other mechanisms are still poorly understood. CIN is typically defined as an increase in serum creatinine by either ≥0.5 mg/dl or by ≥25% from baseline within the first 2–3 days after contrast administration. Although rare in the general population, CIN has a high incidence in patients with an underlying renal disorder, in diabetics, and the elderly. The risk factors are synergistic in their ability to produce CIN. The best way to prevent CIN is to identify the patients at risk and to provide adequate peri-procedural hydration. The role of various drugs in prevention of CIN is still controversial and warrants future studies. Despite remaining uncertainty regarding the degree of nephrotoxicity produced by various contrast agents, in current practice non-ionic low-osmolar contrast media are preferred over the high-osmolar contrast media in patients with renal impairment. © 2007 Wiley-Liss, Inc.
INTRODUCTION
Increasing use of contrast media during radiological procedures has resulted in an increasing incidence of contrast-induced nephropathy (CIN), an iatrogenic disorder caused by exposure to contrast material.
CIN is a complex syndrome of acute renal failure occurring after the administration of iodinated contrast media. The definition includes absolute or relative increase in creatinine level after exposure to contrast agent compared with baseline value, when alternative explanations for renal impairment have been excluded. It occurs within 24–48 hr of the exposure, with creatinine level typically peaking 3–5 days after procedure and returning to baseline or near baseline value in 1–3 weeks [1]. The cut-off increase in creatinine defining CIN differs in various studies (from 20 to 50% or in absolute values from 0.5 to 1.0 mg/dL), making it difficult to compare the results. The most common definition used lately is ≥25% relative increase or an absolute increase of ≥0.5 mg/dL in serum creatinine from baseline value at 48 to 72 hr after exposure to contrast media. On the basis of this definition, the overall incidence of CIN in the general population is reported to be 1.2 to 1.6% [2, 3]. The incidence of CIN is even higher in selected subsets of patients with cardiovascular pathology, which is not surprising given the high prevalence of risk factors for CIN in this population. On the basis of the data registry of the Mayo Clinic including 7,586 patients who underwent percutaneous coronary interventions (PCI), the incidence of CIN was 3.3% [4]. In a smaller study of McCullough et al. [5] that analyzed data on 1,826 patients undergoing PCI, CIN occurred in 14.5% of the cases. Dialysis as a result of CIN in these two series was required in 0.7% and 0.3% of patients, respectively.
PATHOGENESIS OF CIN
The pathogenesis of CIN is not clearly understood. Thus far, several pathophysiological mechanisms of CIN have been proposed, including direct toxicity to renal tubular epithelium, oxidative stress, ischemic injury, and tubular obstruction [6, 7]. Low blood flow in the medulla leading to medullary hypoxia might result from increased perivascular hydrostatic pressure, increased intratubular pressure secondary to contrast-induced diuresis, vasoconstriction due to redundance of vasoactive substances as adenosin and endothelin, and decrease of nitric oxide and prostaglandins [8, 9]. Excretion of the contrast medium requires significant urine volume to clear the osmotic load. Exposure of renal tissues to high osmotic loads results in characteristic histopathologic changes called “osmotic nephrosis.” Changes consistent with osmotic nephrosis were observed in 22.3% of patients undergoing renal biopsy within 10 days of contrast exposure [10]. After injection of contrast media, a transient increase is followed by a more prolonged decrease in renal blood flow in animals and humans [11]. Endothelin-1 has been implicated as the most likely causative agent in a number of studies [12, 13]. The vasoactive effect of adenosine in different organs is dependent on the ratio of adenosine A1 and A2 receptors. In kidneys, in contrast to heart, adenosine causes vasoconstriction and is also thought to play a role in pathogenesis of CIN due to increase of renal adenosine concentrations as a result of enhanced adenosine triphosphate hydrolysis [14]. Reactive oxygen species, which are generated during hypoxia, also probably contribute to renal injury [15].
RISK FACTORS OF CIN
Risk factors for the development of CIN have been thoroughly examined in several studies and are summarized in Table I. They may be divided into two categories: fixed (nonmodifiable) and modifiable.
| Fixed (nonmodifiable) risk factors | Modifiable risk factors |
|---|---|
| Age | Volume depletion |
| Preexisting renal failure | Volume of contrast media |
| Diabetes mellitus | Nephrotoxic drug use (NSAID, cyclosporine, aminoglycosides, cisplatinum) |
| Congestive heart failure | Low serum albumin level (<35 g/L) |
| Hemodynamic instability | Anemia |
| Nephrotic syndrome | |
| Renal transplant |
- NSAID, non-steroidal anti-inflammatory drug.
The best recognized nonmodifiable risk factors include older age, diabetes mellitus, preexistent renal insufficiency, congestive heart failure, hemodynamic instability, and nephrotic syndrome.
Age
The elderly are at increased risk of CIN with reported incidence of 11% in patients older than 70 years [3]. The reasons for higher risk of developing CIN in the elderly have not been studied specifically and probably are multifactorial, including age-related change in renal function as diminished glomerular filtration rate (GFR), tubular secretion and concentration ability, as well as more difficult vascular access requiring greater amount of contrast, presence of multivessel disease, etc. Importantly, by multivariate analysis, age older than 70 years appeared to be an independent predictor of CIN in some studies [16-18].
Preexisting Renal Disease
Preexisting renal disease with an elevated level of creatinine is a crucial risk factor in the development of CIN; rates in patients with underlying renal disorder are extremely high, ranging from 14.8 to 55% [4, 5, 19]. In multivariate analysis, baseline creatinine represented an independent predictor of CIN in the majority of the studies [3-5, 19]. In contrast, the risk of CIN is minimal (<10%) in patients who have normal renal function at the time of contrast-medium exposure.
Higher baseline creatinine values are associated with greater risk of CIN [20]. As shown in study by Hall [21] if baseline plasma creatinine level is ≤1.2 mg/dL, the incidence of CIN was only 2%. However, in patients with values of creatinine in the range of 1.4–1.9 mg/dL, the incidence of CIN increased to 10.4%, and in patients with baseline creatinine level ≥2.0 mg/dL, 62% developed CIN after angiography. A model that predicted CIN by the serum creatinine level showed an exponential increase in the risk for nephrotoxicity if the baseline level was 1.2 mg/dL or higher [22]. Generally, estimated GFR <60 mL/min/1.73 m2 is considered a cut-off value for increased risk of CIN[23].
Diabetes Mellitus
Diabetes mellitus has been identified as an independent risk factor for CIN in numerous studies [3-5, 24]. The incidence of CIN in diabetics varies from 5.7 to 29.4% [2, 25, 26]. Given the high prevalence of diabetes in the general population and its ability to cause broad spectrum of cardiovascular diseases, which require radiological procedures for their diagnosis and treatment, diabetic patients represent a significant proportion of those undergoing contrast exposure. Risk of CIN is increased even in diabetics with preserved renal function [24, 27]. Presence of other risk factors, such as renal insufficiency or proteinuria, in diabetics further increases the risk for CIN. In study by Berns et al. [1], CIN occurred in 27% of diabetics with baseline serum creatinine 2.0 to 4.0 mg/dL and in 81% of those with serum creatinine >4.0 mg/dL. In a study by Toprak et al. [28], a total of 421 patients with Cockcroft–Gauldt estimated creatinine clearance between 15 and 60 mL/min were divided into three groups: diabetes mellitus (n = 137; glucose ≥ 126 mg/dL), pre-diabetes (n = 140; glucose between 100 and 125 mg/dL), and normal fasting glucose (n = 144; glucose < 100 mg/dL). CIN, defined as an increase of ≥25% in creatinine over baseline within 48 hr of angiography, occurred in 20% of diabetics, 11.4% of pre-diabetics, and 5.5% of patients with normal fasting glucose level.
Congestive Heart Failure and Hemodynamic Instability
Since reduced renal perfusion is probably a major mechanism of renal injury in CIN, it is not surprising that several clinical situations associated with hemodynamic impairment were shown to predispose to CIN. Congestive heart failure has been associated with increased risk for CIN in several studies [3, 4, 24, 29]. Anterior myocardial infarction as well as indicators of hemodynamic instability, such as periprocedural hypotension and use of an intra-aortic balloon pump, were shown to be predictors of CIN in patients undergoing primary PCI [29, 30].
Renal Transplant
Concomitant use of nephrotoxic drugs (cyclosporine) along with higher prevalence of diabetes and renal insufficiency results in high risk of CIN in patients with renal transplant. Ahuja et al. [31] retrospectively assessed the data on 144 patients with functioning renal allograft who were exposed to contrast media. The incidence of CIN was 21.2% in the whole group, and was especially high (42.8%) among those who have not received hydration before the procedure.
Volume of Contrast Media
Volume of contrast media administered during the procedure is of primary importance in the development of CIN [26]. It is a main modifiable risk factor for CIN. However, growing complexity of coronary procedures inevitably causes an increased use of contrast media per procedure and consequently enhances the risk of CIN. The correlation between the amount of contrast and the risk of CIN was documented in a number of studies [32, 33]. According to McCullough et al. [5], the risk of CIN is minimal in patients receiving <100 mL of contrast media.
Nephrotoxic Drugs
It is anticipated that concomitant use of nephrotoxic drug and contrast administration will increase risk of CIN. Alamartine reported a trend toward a higher incidence of CIN (P = 0.07) in patients receiving nephrotoxic drugs (including diuretics, nonsteroidal anti-inflammatory drugs, coxibs, aminoglycosides, amphotericin B) [34]. It is a common clinical practice to avoid any other nephrotoxic insults, if it is feasible, when contrast medium is administered.
The contribution of angiotensin-converting enzyme (ACE) inhibitors to risk of CIN is still controversial. In a study by Kini et al. [17], patients receiving ACE inhibitors had a significant increase in serum creatinine after the procedure compared with patients without this therapy. Similarly, in study by Cirit [35], patients with renal insufficiency receiving ACE inhibitors had a higher incidence of CIN after contrast administration than patients who did not receive ACE inhibitors (15.6% vs. 5.8%; P = 0.015). However, another study showed that preprocedure ACE inhibitor use was associated with a lower risk for CIN in patients with chronic renal disease (OR, 0.61; P = 0.005) [24]. Similarly, another clinical trial showed that periprocedural captopril reduced the risk for CIN, compared with an untreated control group [36].
Anemia
In a large registry of 6,773 consecutive patients treated with PCI, low baseline hematocrit was identified as an independent predictor of CIN by multivariate analysis [27]. CIN (increase of ≥25% or ≥0.5 mg/dL over preprocedure serum creatinine, at 48 hr postprocedure) rates steadily increased with baseline hematocrit quintile decrements (from 10.3% in the highest quintile to 23.3% in the lowest quintile) (P for trend < 0.0001).
Type of Contrast Agent
Despite the structural similarity of currently used contrast media (all of them represent derivatives of bensoic acid), there are substantial differences in the chemical properties of these various agents, including the number of iodine molecules, sodium content, and osmolar composition. These latter properties define such characteristics of contrast media as osmolarity, ionicity, and viscosity. Properties of contrast media are listed in Table II.
| Generic name | Osmolarity | Ionicity |
|---|---|---|
| Diatrizoate | High-osmolar | Ionic monomer |
| Iothalamate | High-osmolar | Ionic monomer |
| Ioxithalamate | High-osmolar | Ionic monomer |
| Ioxaglate | Low-osmolar | Ionic dimer |
| Iohexol | Low-osmolar | Nonionic monomer |
| Iopamidol | Low-osmolar | Nonionic monomer |
| Ioversol | Low-osmolar | Nonionic monomer |
| Iopromide | Low-osmolar | Nonionic monomer |
| Iobitridol | Low-osmolar | Nonionic monomer |
| Iomeprol | Low-osmolar | Nonionic monomer |
| Iodixanol | Iso-osmolar | Nonionic dimer |
Numerous studies comparing different contrast agents have been conducted. Barrett et al. [37] published in 1993 a meta-analysis of 31 randomized trials comparing low-osmolality contrast media and high- osmolality contrast media. Pooled odds of a rise in serum creatinine level of more than >0.5 mg/dL with low-osmolality contrast media was 0.61 (95% confidence interval [CI], 0.48–0.77) times that after high-osmolality contrast media. The effect of low-osmolality contrast media in reducing the risk of a rise in serum creatinine of >0.5 mg/dL was significant in patients with renal impairment (OR 0.5; CI, 0.36–0.68) but not in those with normal renal function (OR 0.75; CI, 0.52–1.1). The authors concluded that use of low-osmolality contrast media may be beneficial in patients with existing renal failure [37]. These finding were confirmed in a prospective, randomized, double-blind multicenter trial by Rudnick et al. [19] comparing low-osmolar nonionic contrast agent, iohexol, and the high-osmolar ionic contrast agent, diatrizoate, in 1,196 patients undergoing cardiac angiography. Acute nephrotoxicity (increase in serum creatinine of ≥1 mg/dL, at 48 to 72 hr postprocedure) was observed in 7% of patients receiving diatrizoate compared with 3% of patients receiving iohexol (P < 0.002). Differences in nephrotoxicity between the two contrast groups were confined to patients with previous renal insuficiency or renal insufficiency combined with diabetes mellitus.
A pooled analysis of 16 double-blind, randomized, controlled trials (n = 2,727) comparing nephrotoxicity of iso-smolar contrast medium iodixanol with low-osmolar contrast media was recently published [38]. The maximum creatinine increase within 3 days after contrast medium administration was significantly smaller in the iodixanol group compared with the low-osmolar contrast media group (0.06 mg/dL vs. 0.10 mg/dL; P < 0.001). CIN, defined as an increase in creatinine ≥0.5 mg/dL within 3 days after contrast media administration, occurred less frequently in the iodixanol group than in the low-osmolar contrast media group in all patients (1.4% vs. 3.5%, P < 0.001), in renal insuficiency patients (2.8% vs. 8.4%, P = 0.001), and in patients with combination of renal insufficiency and diabetes mellitus (3.5% vs. 15.5%, P = 0.003). In recently published RECOVER [39] and ICON [40] trials, patients with chronic renal insufficiency were randomly assigned either to iso-osmolar contrast media iodixanol or low-osmolar contrast media ioxaglate. The incidence of CIN, defined as ≥25%, or ≥0.5 mg/dL increase of creatinine, was significantly lower with iodixanol (7.9%) than with ioxaglate (17.0%; P = 0.021) in RECOVER trial [39], but there was no significant difference between both group (16.2% vs. 24.2%, respectively; P = 0.285) in ICON trial [40].
Generally, use of non-ionic low-osmolar contrast media leads to lower rates of CIN than the high-osmolar contrast media, especially in patients with renal impairment. Recent data suggests that use of the iso-osmolar iodixanol is associated with smaller rises in creatinine and lower rates of CIN than low-osmolar contrast media, especially in patients with renal insufficiency and with combination of renal insufficiency and diabetes mellitus [38, 39].
Cumulative Risk Assessment
Risk factors for development of CIN usually occur in combination in individual patients. Mehran et al. developed single risk score for prediction of CIN in patients after PCI [29]. Algorithm for assessment of risk of CIN is shown in Figure 1. Bartholomew et al. [41] suggested another score system based on 8 variables associated with CIN: creatinine clearance <60 mL/min, use of intra-aortic balloon pump, urgent coronary procedure, diabetes mellitus, congestive heart failure, hypertension, peripheral vascular disease, and contrast volume.
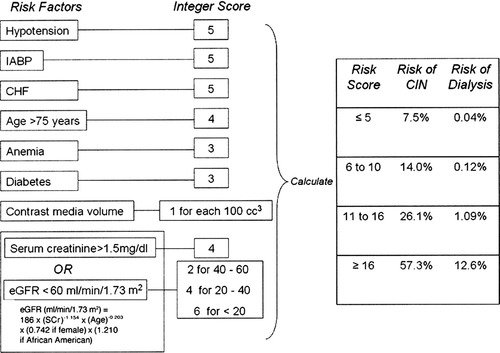
Risk score for prediction of contrast-induced nephropathy by Mehran et al. IABP, intra-aortic balloon pump; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate.
PROGNOSIS OF CIN
Today, CIN is one of the most common sources of acute renal failure among hospitalized patients. It is associated with prolonged in-hospital stay and increased morbidity, mortality, and costs. Previous studies have shown that 12–14% of patients who develop acute renal insufficiency during hospitalization do so after procedures involving radiographic contrast [5, 42]. A retrospective analysis of 16,248 patients exposed to contrast media showed that even apparently small decreases in renal function can lead to excessive mortality rates independent of other risk factors, and given that small rises in serum creatinine levels actually represent a significant drop in GFR [43]. In-hospital mortality rates were almost fivefold higher in patients that developed CIN (34%) compared with those without renal failure (7%) [43]. Prognosis is especially unfavorable in patients with preexisting renal disease, in whom contrast material causes further deterioration of renal function, and those on dialysis [44]. In-hospital mortality in these subsets was 14.9% and 27.5%, respectively, versus 4.9% in patients with preserved renal function [44, 45]. In the Mayo Clinic registry, in-hospital mortality in patients undergoing PCI and developing CIN was 22% compared with only 1.4% in patients without CIN [4]. In-hospital mortality is especially high (36%) in patients that require dialysis after the radiocontrast procedure [5].
During the first year after exposure to contrast, rates of mortality in patients with underlying renal disease remain very high, being 45.2% in patients requiring dialysis, 35.4% in patients with deterioration of renal function, and 19.4% in those with stable renal function [44]. According to the data of the Mayo Clinic PCI registry, 1-year mortality correlates directly with creatinine clearance, being 1.5% in individuals with creatinine clearance ≥70 mL/min and 18.3% in patients with creatinine clearance ≤30 mL/min [46].
PREVENTION OF RADIOCONTRAST NEPHROPATHY
Creatinine level measurements should be performed before angiography in patients with a history of kidney disease, proteinuria, kidney surgery, diabetes, hypertension, or gout [47]. The creatinine clearance rate or the GFR should be estimated from the serum creatinine level, according to the Cockcroft–Gault formula [48], to identify more accurately those patients with values below 60 mL/min/1.73 m2, who are at increased risk for nephropathy. Alternative imaging methods not requiring contrast medium should be considered in patients with risk factors. Serum creatinine levels should be measured 24–48 hr after administration of the contrast medium.
The unfavorable prognostic implications of CIN make its prevention of paramount importance. Thus far, several potential risk-reduction strategies have been investigated.
Hydration
There is a strong consensus that adequate volume expansion prior to administration of contrast media is a major strategy in prevention of CIN, though no randomized controlled trials directly comparing a strategy of volume expansion with no volume expansion have been carried out to date. Several potential mechanisms can contribute to the beneficial effect of volume expansion, including dilution of contrast media within the tubule lumen, reduced activation of renin-angiotensin system due to increased delivery of sodium to the distal nephron, and minimizing reductions in the renal production of nitric oxide caused by contrast media [23].
The positive effect of adequate hydration in reducing rates of CIN was established in the randomized study of Solomon et al. [49]. In 78 patients with chronic renal insufficiency undergoing cardiac angiography, hydration with 0.45% saline 12 hr before and 12 hr after angiography provided better protection against renal function deterioration than did hydration with 0.45% saline plus mannitol or saline plus furosemide (CIN 11% vs. 28% vs. 40%; P = 0.05). In the randomized PRINCE trial [50], the achievement of high urine flow rate by forced diuresis with intravenous 0.45% saline, mannitol, and furosemide provided, compared with control (0.45% saline plus placebo), only modest benefit in prevention of CIN. Two randomized studies compared different modes of fluids administration: intravenous versus oral [51, 52]. While the first study of 36 patients comparing 0.45% saline solution intravenously for 24 hr with 1,000 mL liquid orally preprocedure followed by 6-hr saline infusion did not demonstrate a difference between two arms (CIN 11.1% vs. 5.6%, respectively; P = NS) [51], the study by Trivedi (53 patients) favored intravenous administration of fluids (CIN 3.7% vs. 34.6%, respectively; P = 0.005) [52]. Two small studies compared overnight and short bolus infusion prior to catheterization. The first study showed a significantly higher decline in GFR in the bolus group (delta GFR 34.6 ± 25.7 mL/min/1.73 m2) compared with patients receiving the overnight prehydration regimen (delta GFR 18.3 ± 25.0 mL/min/1.73 m2; P < 0.05) [53], while the second study did not show significant difference between groups [54].
The randomized, open-label study of Mueller et al. [55] raised the question of which solution is better for hydration in prevention of CIN. The comparison of two saline solutions of varying tonicity in a total of 1,620 patients undergoing coronary angioplasty showed the superiority of isotonic (0.9% saline) versus half-isotonic (0.45%) saline in reducing rates of CIN (0.7% vs. 2%, respectively; P = 0.04). The benefit of isotonic saline was especially prominent in women, diabetics, and patients receiving more than 250 mL of contrast media. The better efficacy of normal than half-normal saline can be explained by its enhanced ability to expand intravascular volume. A prospective, randomized single-center study of 119 patients suggested that hydration with isotonic sodium bicarbonate (154 mEq/L) 1 hr before and 6 hr after administration of contrast agent iopamidol is more effective than hydration with sodium chloride for prophylaxis of CIN (1.7% vs. 13.6%, respectively; P = 0.02) [56]. It has been speculated that alkalinizing the urine reduces the nephrotoxicity of iodinated contrast media through changes in redox potential or through decreasing the viscosity of the agents within the vasa recta. Since this was relatively small study with significant dropouts from follow-up, larger, randomized, multicenter study is warranted to evaluate efficacy of this simple inexpensive method in preventing CIN.
CIN Consensus Working Panel recommendations were published recently in the American Journal of Cardiology. After careful revision of available information from performed studies, they suggest adequate intravenous volume expansion with isotonic crystalloid (1.0–1.5 mL/kg/hr) for 3 to 12 hr before the procedure and continued for 6 to 24 hr to prevent development of CIN in patients at risk [57]. Caution is needed in patients with chronic heart failure. They can profit more from optimal hemodynamic stabilization than excessive hydration.
Acetylcysteine
The use of acetylcysteine, an agent with antioxidant properties, in the prevention of CIN is based on the assumption that CIN is caused by reactive oxygen species, formed as a result of a direct toxic effect of contrast media on tubular epithelial cells. In a randomized placebo-controlled study of 83 patients exposed to contrast media, prophylactic administration of acetylcysteine along with hydration was superior to hydration alone in prevention of CIN in patients with elevated baseline creatinine level (CIN 2% vs. 21%, respectively; P = 0.01) [58]. These results caused significant enthusiasm among physicians, and acetylcysteine along with hydration has been adopted by many centers as a standard preparation for contrast exposure, especially in high-risk patients. Subsequently, the Acetylcysteine to Prevent Angiography-Related Renal Tissue Injury trial [59], including 54 patients and using a similar design, confirmed the previous results: CIN occurred in 8% of patients in the acetylcysteine group versus 45% in the placebo group (P = 0.005).
Lately, however, enthusiasm regarding the efficacy of acetylcysteine has been diminished, since several studies did not show a significant benefit of acetylcysteine in comparison to controls (Table III) [60-65]. Since administration of a standard dose of acetylcysteine (600 mg bid orally) did not result in the expected reduction of CIN, several investigators used higher doses of acetycystein. In study by Briguori et al. [66] involving patients with chronic renal failure and comparing standard (600 mg) and high (1,200 mg) doses of acetylcysteine orally twice daily on day of procedure, the rate of CIN was lower in patients receiving the high dose (4% vs. 11%; P = 0.03). The benefit of high-dose acetylcysteine was even more pronounced in patients who received a larger volume of contrast medium (≥140 mL). In the Rapid Protocol for the Prevention of Contrast-Induced Renal Dysfunction trial [67], patients with mild-to-moderate chronic renal failure undergoing elective coronary interventions received intravenous acetylcysteine at a dose of 150 mg/kg before procedure and a dose of 50 mg/kg over the following 4-hr period. The Rapid Protocol for the Prevention of Contrast-Induced Renal Dysfunction trial showed a significantly lower incidence of CIN in treated patients as compared with controls (5% vs. 21%; P = 0.04).
| Author of study | N | Inclusion criteria | Active arm—dose of acetylcysteinea | CIN definition | CIN incidence (%) | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 0 | Day 1 | Acetylcysteine | Control | |||||
| Azmus [61] | 397 | Cr ≥1.3 mg/dL, DM or ≥70 yr | 600 mg bid | 600 mg bid | 600 mg | ≥25% or ≥0.5 mg/dL increase in SCr at 48 hr | 7.1 | 8.4 | 0.62 |
| Boccalandro [62] | 179 | SCr >1.2 mg/dL or CrCl <50 mL/min | 600 mg bid | 600 mg bid | ≥0.5 mg/dL increase in SCr at 48 hr | 13 | 12 | 0.84 | |
| Briguori [66] | 183 | SCr >1.2 mg/dL and/or CrCl <70 mL/min | 600 mg bid | 600 mg bid | ≥25% increase in SCr at 48 hr | 6.5 | 11 | 0.22 | |
| Kay [64] | 200 | SCr >1.2 mg/dL or CrCl <60 mL/min | 600 mg bid | 600 mg bid | ≥25% increase in SCr at 48 hr | 4 | 12 | 0.03 | |
| Marenzi [60] | 354 | ST- elevation myocardial infarction | 600 mg bid vs. ddb | 600 mg bid vs. ddb | 600 mg bid vs. ddb | ≥25% increase in SCr at 72 hr | 15 vs. 8b | 30 | <0.001 |
| Webb [65] | 487 | GFR <50 mL/min | 500 mg iv | >5 mL/min decrease in CrCl | 23.3 | 20.7 | 0.57 | ||
- CIN, contrast-induced nephropathy; CrCl, creatinine clearance; IV, intravenous; SCr, serum creatinine; dd, double dose.
- a Administered orally unless otherwise indicated.
- b Three-arm study: standard-dose vs. high-dose vs. placebo.
Meta-analyses of acetylcysteine trials generally conclude that acetylcysteine may reduce CIN, but trials are very heterogeneous and results are inconsistent [68, 69]. Further investigation is warranted.
Dopamine
Given its dilatory effect on the renal vasculature and the ability to increase renal blood flow and GFR, dopamine was supposed to be useful in the prevention of CIN. This hypothesis was evaluated in several studies and the results turned out to be conflicting. Dopamine was shown to attenuate the increase in creatinine level after exposure to contrast media in one study [70], while in others, such effect was not documented at all [71], or was present only in patients with creatinine ≥2.0 mg/dL [72]. Moreover, in patients with peripheral vascular disease and CIN the effect of dopamine on renal function was found to be deleterious [70, 73].
Fenoldopam
Fenoldopam, a selective, dopamine-1 receptor agonist known to produce both systemic and renal arteriolar vasodilatation, was shown to blunt the decline in renal blood flow and GFR in animals exposed to contrast media [74]. Observational studies reported rather low rates of CIN in high-risk patients treated with fenoldopam [17, 75, 76]. In a double-blind, randomized, placebo-controlled pilot trial [77], the combination of fenoldopam and hydration, compared with hydration alone, resulted in an increase in renal plasma flow, a decrease in peak serum creatinine level 72 hr after exposure to contrast media, and a trend toward decreased incidence of CIN (21% and 41%, respectively; P = 0.14). Two other prospective randomized trials showed negative results [78, 79]. In the first trial, patients were randomized to saline infusion alone (control) or saline with fenoldopam (0.1 μg/kg/min for 4 hr before and after the procedure); a third arm was treated with acetylcysteine. The incidence of CIN was similar in the fenoldopam and control groups (15.7% vs. 15.3%, respectively; P = NS) [78]. The second larger trial by Stone et al. [79] confirmed the lack of benefit with fenoldopam. In this double-blind trial, a total of 315 patients (all treated with saline 0.45%) were randomized to fenoldopam (0.05 μg/kg/min titrated to 0.1 μg/kg/min) or placebo starting 1 hr before the procedure and continuing for 12 hr afterward. There was no significant difference in the incidence of CIN within 96 hr in the 2 groups (33.6% vs. 30.1%, respectively; P = NS), or rates of dialysis, rehospitalization, and death at 30 days [79].
Terstein et al. [80] hypothesized that lack of effect could be a consequence of the inability to administer an effective renal dose of fenoldopam and conducted randomized, controlled study using intrarenal administration of fenoldopam via bifurcated renal infusion catheter in patients after coronary angiography. Patients randomized to fenoldopam received initially intravenous fenoldopam and after period of washout they crossed over to intrarenal fenoldopam. Compared with intravenous fenoldopam, intrarenal administration was associated with a significantly higher GFR (73.7 ± 3.1 vs. 62.6 ± 2.5 mL/min, respectively; P = 0.0007). Fenoldopam plasma levels were lower (3.3 ± 0.3 vs. 4.8 ± 0.3 ng/mL, respectively; P < 0.0001) and a major adverse effect, systemic hypotension, was less pronounced with intrarenal administration of fenoldopam (systolic blood pressures 125.5 ± 3.6 vs. 117.4 ± 2.8 mmHg; P < 0.0001).
Theophylline
As mentioned earlier, several studies provided evidence of adenosine involvement in the renal hemodynamic response to contrast media [81, 82]. This raised the hypothesis that adenosine A1-receptor antagonist, theophylline, may attenuate the decrease in renal blood flow and GFR induced by the exposure to contrast media. Several randomized studies were conducted, but results were inconsistent. In a randomized, placebo-controlled study [83], prophylactic intravenous administration of 200 mg theophylline reduced the incidence of CIN in patients with chronic renal insufficiency compared with placebo (4% vs. 16%, respectively, P = 0.046). In a study by Kapoor et al. [84], 70 patients with diabetes mellitus were randomized in two groups. The active arm received theophylline 200 mg bid orally 24 hr before and for 48 hr after coronary angiography. No patient in the theophylline group had a >25% rise in serum creatinine, compared with 20% in the control group (P = 0.017). Incidence of CIN, defined as a ≥25% fall in GFR, was significantly lower in the theophylline group (3% vs. 31%; P = 0.004). In another randomized, placebo-controlled study [85], treatment with 165 mg theophyllin intravenously compared with placebo was accompanied by lesser decrease in GFR, plasma erythropoietin, and renin activity. Two other randomized studies, however, did not show any benefit of theophyllin compared with placebo in preventing CIN [73, 86].
Calcium Channel Antagonists
Following observations of contrast-induced alterations in calcium metabolism and the ability of calcium channel antagonists to relieve vasoconstriction, several studies investigated the effect of calcium channel blockers on rates of CIN. In a small (35 patients) randomized study [87], GFR was preserved in patients treated with nitrendipine but decreased in patients that received placebo (27% on day 2 after contrast administration; P < 0.01). In contrast, in two other studies with nitrendipine and nifedipine [88, 89], the change in creatinine level did not differ significantly between the groups.
Prostaglandin E1
Based on the decreased levels of prostaglandins in patients with CIN, it was hypothesized that prophylactic administration of prostaglandin E1 may be beneficial in reducing CIN [90]. A double-blind, randomized, placebo-controlled study investigated the effect of intravenous administration of prostaglandin E1 in three various doses. All the groups treated with prostaglandin E1, independently of given dose, experienced significantly less increase in creatinine after exposure to contrast media compared with placebo. The most pronounced effect was observed in patients that received the intermediate dose of the drug (20 ng/kg/min) [90].
Ascorbic Acid
In view of the possible role of oxidative stress and free radical production in pathogenesis of CIN, oral ascorbic acid (3 g before and 2 g given 2 times after the procedure) was evaluated in a randomized, double-blind, placebo-controlled trial in 231 patients undergoing cardiac catheterization. The incidence of CIN, defined as ≥0.5 mg/dL or a ≥25% increase in serum creatinine, was 9% in the ascorbic acid group and 20% in the placebo group (P = 0.02) [91].
Atrial Natriuretic Peptide
Atrial natriuretic peptide in three different doses failed to prevent CIN in the randomized, placebo-controlled study of Kurnik et al. [25].
Hemodialysis and Hemofiltration
Several studies examined the effect of hemodialysis, immediately after exposure to contrast media, in preventing renal function deterioration in patients with preexisting renal disease. All these studies provided consistent results, showing that prophylactic hemodialysis does not diminish the rates of CIN [92, 93].
Two studies by Marenzi et al. [94, 95] investigated the effect of continuous venovenous hemofiltration in prevention of CIN in patients with severe chronic renal insufficiency (serum creeatinine >2 mg/dL) in comparison with intravenous hydration. Increase of creatinine >25% (5% vs. 50%, respectively; P < 0.001) and in-hospital mortality (2% vs. 14%, respectively; P = 0.02) were significantly lower in group with hemofiltration. However, since creatinine level is naturally positively influenced by hemofiltration, assessment of benefit in prevention of CIN based on this endpoint is certainly debatable. This method deserves further investigation. Among different mechanisms possibly involved, high-volume controlled hydration before contrast media exposure plays a major role [94].
Withdrawal of Nephrotoxic Drugs and Metformin
Potentially nephrotoxic drugs should be withdrawn before contrast administration in patients at risk for CIN. It is also common practice to withdraw metformin to avoid the risk that metabolic acidosis might be precipitated if a postprocedure decline in renal function occurs.
CONCLUSION
CIN is an iatrogenic disorder, resulting from exposure to contrast media. Contrast-induced direct cytotoxic effect on renal structures, along with deterioration of renal blood flow, are evident in its pathogenesis, while other mechanisms are still poorly understood. Although rare in general population, CIN has a high incidence in patients with underlying renal disorder, diabetics and the elderly. The risk factors are synergistic in their ability to produce renal injury after administration of contrast media. The best way to prevent CIN is to identify the patients at risk and to provide adequate periprocedural hydration. The role of various drugs in prevention of CIN is still controversial and warrants future studies. Despite remaining uncertainty regarding the degree of nephrotoxicity produced by various contrast agents, in current practice non-ionic low-osmolar contrast media are preferred over the high-osmolar contrast media in patients with renal impairment.




