PRKCQ Is Dispensable for Spermatogenesis in Mice
Yu Chen, Hong Chen, and Zhongyan Han contributed equally to this study.
ABSTRACT
Protein kinase C (PKC) family is evolutionally conserved and involved in various signaling cascades in all cells. Of the family, PRKCQ is dominatingly expressed in testis, however, its molecular functionality in spermatogenesis and male fertility remains unclear. To evaluate the role of PRKCQ in spermatogenesis, Prkcq knockout mice were generated using CRISPR/Cas9 system. Histological and immunofluorescence assays by different markers were employed to assess the testicular cells variation. Sperm parameters were analyzed by computer-assisted sperm analyzer. qPCR assay was used to examine the expression levels of other PKC family genes. We found that PRKCQ was conserved throughout evolution and highly expressed in testis. Prkcq−/− mice were successfully generated and developed viably. Normal fertility was observed in Prkcq−/− males. Prkcq−/− mice exhibited no defects in spermatogenic cells and mature sperm were full in epididymis. Furthermore, there were no differences in sperm motility and progressive motility between Prkcq−/− males and controls. Our findings report a detailed phenotypic analysis of Prkcq−/− males and indicate that PRKCQ is not required for spermatogenesis in male mice, which can provide basic information for other researchers.
Abbreviations
-
- BCA
-
- bicinchoninic acid
-
- CASA
-
- computer-assisted sperm analysis
-
- HTF
-
- human tubal fluid
-
- MDF
-
- modified Davidson's fluid
-
- PAS
-
- periodic acid-Schiff's
-
- PKC
-
- protein kinase C
-
- sgRNAs
-
- single guide RNAs
-
- SSCs
-
- spermatogonia stem cells
-
- TNBC
-
- triple-negative breast cancers
-
- TNP1
-
- transition protein 1
1 Introduction
Spermatogenesis is an extraordinary complex and ordered process during which spermatogonia stem cells (SSCs) terminally differentiate to produce mature sperm (Neto et al. 2016). SSCs in the basal of seminiferous tubules can self-renew to maintain lifelong spermatogenesis and a portion of those differentiate to generate differentiated spermatogonia (Guo et al. 2021). The differentiated spermatogonia then undergo cell divisions to yield primary spermatocytes, which represent to enter the meiotic phase and differentiate into round spermatids (Liu et al. 2019). During spermiogenesis, these round spermatids matured into highly specialized sperm through dramatically morphological changes including spermatid DNA condensation, acrosome and flagellar formation and most cytoplasm loss (Wang et al. 2021).
Phosphorylation is known to be one of the most abundant and important posttranslational modifications and involved in the majority of cellular pathways (Manning et al. 2002; Jacob et al. 2011), as well as plays critical roles in regulation of spermatogenesis. For example, Casein kinase 1α (CK1α) deletion leads to male infertility by impairing committed progenitor spermatogonia through p53/Sox3 signaling pathway (Lu et al. 2022). Phosphoproteomic profiling reveals the complicated protein phosphorylation and kinase-substrate network of spermatocytes (Li et al. 2022a), suggesting that protein phosphorylation is essential for meiosis. The serine/threonine-protein kinase ATR plays the central roles in meiosis, phosphoproteomic study found a range of phosphorylation events mediated by ATR (Sims et al. 2022). Moreover, spermiogenesis has been found to be subjected to protein phosphorylation regulation (Li et al. 2019) with enrichment of phosphorylation substrates related to biological process in spermiogenesis. Aurora kinase C (AURKC) mutations in patients cause large-headed polyploid spermatozoa and male infertility (Dieterich et al. 2007; Ben Khelifa et al. 2011).
Kinases are characterized by the ability to catalyze phosphate group to its substrate and serine/threonine protein kinases are most of all the protein kinases (Wu et al. 2024). Protein kinase C (PKC), belongs to the prototypical class of serine/threonine kinases, is considered to be activated by lipid and classified into three types including the conventional type (cPKC; PKCα, β, and γ), the novel type (nPKC; PKCδ, ε, θ, and η), and the atypical type (aPKC; PKCζ and ι) (Takai et al. 1979). All PKC isozymes contain a common architecture of C-terminal kinase domain and the regulatory moiety in N-terminal (Newton 2018a). It has been well-documented that PKC dysregulation is involved in various diseases such as cancers, cardiovascular system diseases and neurological problems (Miao et al. 2022; Garg et al. 2014; Singh et al. 2024). However, the molecular function of PKC in spermatogenesis remains unknown. Only few research report the expression of PKC isozymes in murine testis. In situ hybridization and Northern blot reveal that PKCδ (PRKCD) specifically expressed at the spermatid stage in mice (Um et al. 1995). Furthermore, PKC isozymes exhibit progressive reduction in the early stages of rat spermatogenesis (Zini et al. 1997). Interestingly, PKCθ (PRKCQ) has the unique expression pattern with high mRNA levels in mouse testes (Mischak et al. 1993; Niino et al. 2001), which suggest some related function of PRKCQ in spermatogenesis. However, there is no evidence for confirming the hypothesis.
Here, in our study we used genetic knockout mouse model to explore the potential function of PRKCQ in vivo in spermatogenesis and male fertility. Using CRISPR/Cas9 system, we successfully generated Prkcq knockout mice with 86 bp deletion in the exon 11 of Prkcq gene. Further fertility test showed that Prkcq−/− male were fertility and Prkcq deficiency did not affect the progress of spermatogenesis. Mature sperm from Prkcq−/− mouse displayed normal sperm motility, progressive motility and morphological feature. Collectively, these results indicated that PRKCQ is not essential for spermatogenesis.
2 Results
2.1 Expression Profile Assessment of PRKCQ in Mouse Testis
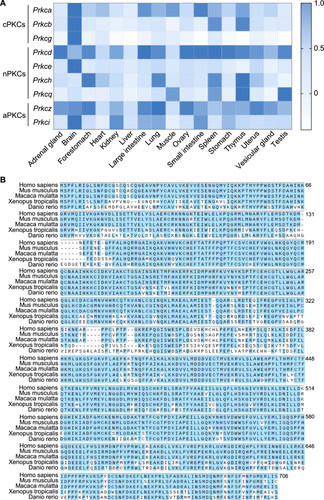
PKC is a family consisting of several phospholipid-dependent serine/threonine kinases and play vital roles in a wide range of cellular functions (Newton 2001, 2018b). Based on their structural and activation characteristics, PKC family is classified into three subfamilies (Dempsey et al. 2000; Steinberg 2008): SSC, spermatogonia stem cells; conventional or classic PKC isozymes (cPKCs; PRKCA, PRKCB, and PRKCG), novel or non-classic PKC isozymes (nPKCs; PRKCD, PRKCE, PRKCH, and PRKCQ), and atypical PKC isozymes (aPKCs; PRKCZ and PRKCI). To investigate the PKC family function in spermatogenesis, we analyzed their expression patterns in various mouse tissues according to the Comprehensive Mouse Transcriptomic BodyMap (Li et al. 2017). As shown in Figure 1A, only Prkcd and Prkcq was preferentially expressed in testis and Prkcq mRNA was expressed highly in testis compared to other tissues. Moreover, PRKCQ is evolutionarily conserved from Danio rerio to Homo sapiens according to multiple sequence alignment analysis (Figure 1B).
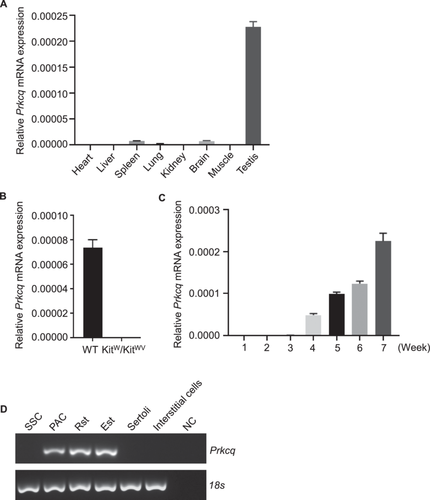
To further explore the role of PRKCQ in spermatogenesis, we first validated that Prkcq mRNA was highly expressed in testis among the eight adult mouse tissues (Figure 2A). Kitw/wv mice only have undifferentiated spermatogonia and normal somatic cells in testis (Ohta et al. 2003) and qPCR analysis showed that Prkcq was not expressed in Kitw/wv mice (Figure 2B). On account of the first round of spermatogenesis is nearly synchronous, postnatal 1-, 2-, 3-, 4-, and 5- to 7-week testes can represent the different types of germ cells corresponding to spermatogonia, early spermatocytes, round spermatids, elongating spermatids and sperm, respectively. We performed qPCR analysis and the results showed that Prkcq begun to express at Week 4 and persisted until Week 7 (Figure 2C). Furthermore, spermatogonia stem cells, pachytene spermatocytes, round spermatids, elongating spermatids, Sertoli cells and interstitial cells were purified and we found that Prkcq was expressed in pachytene spermatocytes, round and elongating spermatids but not in somatic cells (Figure 2D).
2.2 Prkcq−/− Mouse Generation
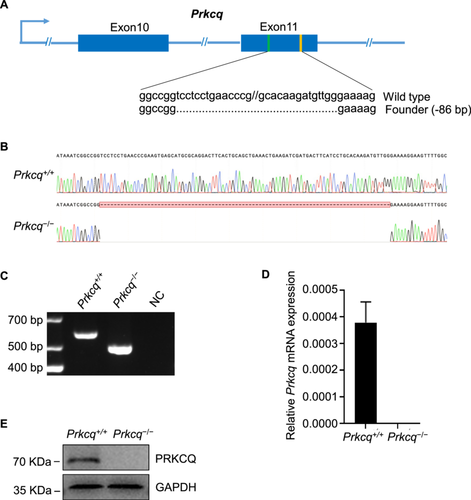
To examine the function of PRKCQ in spermatogenesis, we used CRISPR/Cas9 approach to generate Prkcq−/− mouse with sgRNAs targeting exon 11 (Figure 3A) and obtained 86 bp deletion mutant line validated by sanger sequencing (Figure 3B). Homozygous mutant Prkcq allele were then detected by PCR genotyping (Figure 3C). There was no expression of Prkcq mRNA in Prkcq−/− testis using qPCR analysis (Figure 3D). Western blot further confirmed the absence of PRKCQ protein in Prkcq−/− testis (Figure 3E). Taken together, above results indicated Prkcq knockout mice were successfully generated.
2.3 Prkcq−/− Male Mice Are Fertile and Show Normal Spermatogenesis
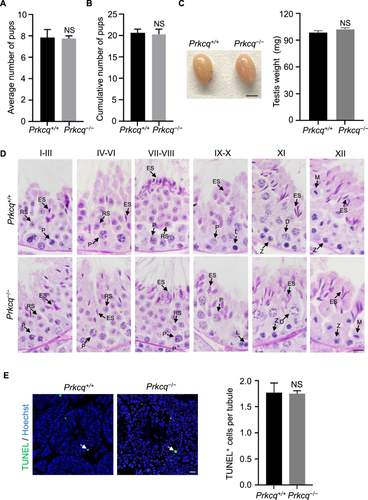
The Prkcq−/− mice were viable and displayed normal behavior. To investigate whether Prkcq deletion affected male fertility, we mated Prkcq−/− male mice with wild-type female mice and the results showed that litter sizes of Prkcq−/− males were no significantly difference compared to Prkcq+/+ males (Figure 4A,B). Also, no difference was observed in testis appearance and testis weight in Prkcq−/− mice (Figure 4C). In addition, compared to Prkcq+/+ males, the PAS-stained Prkcq−/− testis section showed normal spermatogenic cells in seminiferous tubules and did not display any differences in spermatogenesis (Figure 4D). Using TUNEL assay, we then examined the apoptotic state of spermatogenic cells in Prkcq−/− mice and found that the average number of TUNEL positive cells per tubule was similar to that in Prkcq+/+ testis (Figure 4E).
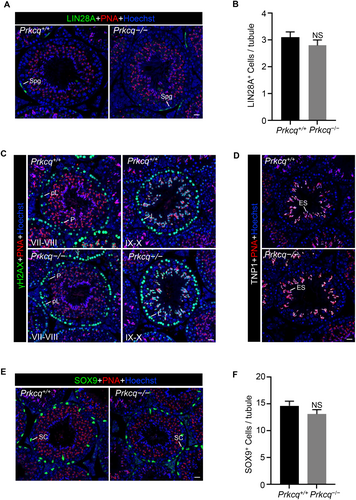
To further characterize the spermatogenesis in Prkcq−/− mice, we performed immunostaining by LIN28A (spermatogonia marker) and revealed that the numbers of LIN28A-positive spermatogonia did not differ between Prkcq+/+ and Prkcq−/− testis (Figure 5A,B). Next, immunofluorescent staining showed similar expression of γH2AX (spermatocyte marker) in Prkcq−/− spermatocytes compared to the controls, suggesting that meiotic progression was not affected after deletion of Prkcq (Figure 5C). We examined the nuclear condensing using transition protein 1 (TNP1) immunofluorescent staining and found that nuclear condensation was normal in Prkcq−/− spermatids (Figure 5D). We then immunostained testis sections for SOX9 (Sertoli cell marker) and the results showed that Prkcq+/+ and Prkcq−/− mice contained similar numbers of Sertoli cells in seminiferous tubules (Figure 5E,F). Altogether, these results indicated that Prkcq deficiency did not affect spermatogenesis.
2.4 Sperm Parameters Are Normal in Prkcq−/− Mice
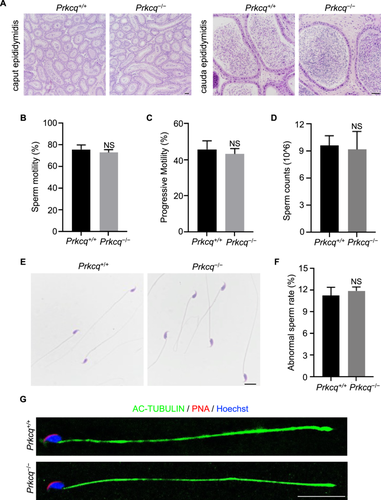
Hematoxylin and eosin (H&E) stained Prkcq−/− mice caput epididymis exhibited normal histology morphology and cauda epididymis was full of mature sperm in Prkcq−/− mice (Figure 6A). Subsequently, we detected the sperm parameters of sperm collected from cauda epididymis using computer-assisted sperm analysis (CASA). As shown in Figure 6B,C, sperm motility and progressive motility from Prkcq−/− mice were similar with those of Prkcq+/+ mice. Additionally, the number of sperm were also comparable between Prkcq+/+ mice and Prkcq−/− mice (Figure 6D). We next analyzed the histology morphology of sperm via H&E staining and found that Prkcq−/− sperm exhibited normal morphology with sickle-shaped head and tail (Figure 6E). The percentage of abnormal sperm was also no significantly difference in Prkcq−/− mice compared with Prkcq+/+ mice (Figure 6F). Consistent with the H&E stained images, immunofluorescent staining suggested that the structures of nuclear, acrosome, and flagella remained normal in Prkcq−/− sperm (Figure 6G).
2.5 No Transcriptional Compensation Observed for Other PKC Isozyme Members in Prkcq−/− Testis
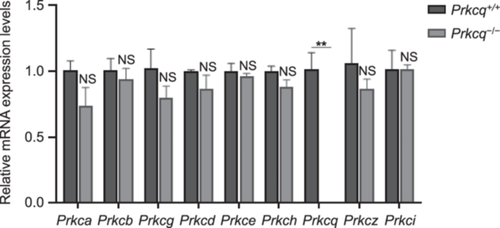
The genetic compensation response has been a possible explanation for the phenotypic absence in gene-knockout mouse. Previous studies showed that compensatory upregulation of the genes is specifically triggered by mRNA bearing a premature termination codon (PTC) and accompanied by an enhancement of H3K4me3 at the transcription start site regions of the compensatory genes (Ma et al. 2019; Rossi et al. 2015). On account of the homology between PKC family components, we further investigated the mRNA levels of other PKC isozymes to explore the effect of Prkcq deletion. The results showed that the mRNA expression levels of other PKC isozymes were not significantly increased in Prkcq−/− testis when compared with that in the testis of controls (Figure 7), indicating that the loss of PRKCQ function was not compensated by other PKC family members.
3 Discussion
Here, we screened PRKCQ, a member of PKC family, as a potential regulator in spermatogenesis and male fertility by analyzing the expression patterns of PKC isozymes in various organs. Unlike most PKC isoforms, we found that Prkcq showed a relatively narrow range of expression in testis and exhibited specifically expression in germ cells. However, further Prkcq knockout mouse did not display abnormal reproductive events. All types of spermatogenic cells existed and functioned well in different stages of spermatogenesis in Prkcq−/− mice. Sperm count and motility were comparable in Prkcq−/− mice when compared with Prkcq+/+ mice.
PRKCQ is previously reported to involve in several cellular pathways and a range of diseases. Most studies concentrated on its roles in T-cell activation and survival. Prkcq deficiency in T cells leads to proliferation impairments, reduction in interleukin 2 (IL-2) and CD25 expression (Sun et al. 2000; Pfeifhofer et al. 2003). PRKCQ is also found to function in cancers and regulate tumor development and progression. For example, PRKCQ is highly expressed in triple-negative breast cancers (TNBC) and upregulate the expression of Fra-1 to promote their invasion (Byerly et al. 2020), thus PRKCQ inhibitor can be a potential antineoplastic drug. Moreover, PRKCQ can regulate the platelet activation and aggregation by participating αIIbβ3 integrin receptors signal transduction pathway (Soriani et al. 2006). In our study, Prkcq−/− males are fertile which is consistent with previous research (Sun et al. 2000). Normal spermatogenesis and no spermatogenic cells defects were found in Prkcq knockout males, suggesting its less important role in testis. Similarly, Prkcd, the other PKC isozyme expressed in testis, knockout mice also develop normally and are fertile (Leitges et al. 2001). Our results also showed that other PKC isozymes exhibited normal expression in Prkcq−/− males. It is likely that there are some other compensatory effects to maintain the normal reproduction process, but it needs further investigation.
It's worth noting that Prkcq−/− mice showed normal spermatogenesis and fertility under strict laboratory conditions. However, external stress factors, such as heat stress, may induce more severe testicular damage, impaired spermatogenesis and increase the risk of male infertility (Chen et al. 2024; Bailey et al. 2002). Therefore, whether Prkcq deletion could result in subtle phenotypes under stress conditions need to be explored in the future.
In summary, we found that PRKCQ, a conserved and germ cell-specific gene, was not required for spermatogenesis and male fertility. This phenotypic report could provide basic information for further studies.
4 Materials and Methods
4.1 Animals
All mice were housed under specific-pathogen-free animal (SPF) conditions in the Animal Core Facility of Nanjing Medical University. Animal care and experimental procedures were designed according to the guidelines and regulations of the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University, and approved by the Animal Ethical and Welfare Committee (No. IACUC-1810007-2).
4.2 Generation of Prkcq Knockout Mice
CRISPR/Cas9 technology was used to generate the Prkcq knockout mice as described previously (Li et al. 2022b). Briefly, the single guide RNAs (sgRNAs) were designed and synthesized to target exon 11 of Prkcq gene. Cas9 mRNA and sgRNAs were then transcribed in vitro and then coinjected into fertilized eggs to obtain the founders. The F0 mice underwent serial mating to generate homozygous mutants following genotyping.
4.3 Genotyping
Tail DNA was extracted using One Step Mouse Genotyping Kit (Vazyme). The genotypes of the offspring were confirmed by PCR amplification (Forward primer, 5′- CCTGGAACTTGCTCTGTAGACTG -3′; Reverse primer, 5′- TGTTGGATGCTCTGTCATCTCAC -3′). Sanger sequencing was additionally conducted and analyzed by SnapGene.
4.4 Testicular Cells Isolation
Interstitial cells and Sertoli cells were collected as described previously (Li et al. 2022b). In brief, followed by 1 mg/mL collagenase IV and 25% trypsin with DNase I at 37°C, the testes were digested into single-cell suspensions and interstitial cells were collected from the supernatant. For the collection of Sertoli cells, single-cell suspensions of adult testes were cultured in cell culture dishes for 24–48 h with changing the medium every 12 h. Sertoli cells adhering to the bottom of the dish were collected for subsequent experiments.
The STA-PUT method (Bryant et al. 2013) was used to isolate pachytene spermatocytes, round spermatids, and elongated spermatids from adult mouse testes, and spermatogonia from postnatal Day 8 mouse testes. Briefly, the single-cell mouse testes suspensions digested by two-step process were subjected to a cell separation device (ProScience Inc. Canada) and separated by a 2%–4% bovine serum albumin (BSA) gradient. After 2.5 h sedimentation, spermatogenic cells were collected for later analysis.
4.5 qPCR
Total RNA was extracted by RNAiso Plus (Takara) and determined the concentration and purity by absorbance at 260/280 nm using a NanoDrop 2000 (Thermo Fisher Scientific). RNA was then reverse-transcribed into cDNA by PrimeScriptRT Master Mix (Takara). The cDNA was subsequently diluted and used for quantitative RT-PCR (qPCR) with SYBR Green Fast qPCR Mix (Abclonal). Primers were provided in Table S1. The QuantStudio 7 (Applied Biosystems) system was used to conduct gene expression levels and analyzed by the comparative ΔΔCt (cycle threshold) method according to the manufacturer's instruction.
4.6 Western blot
Testis tissues were separated from adult Prkcq+/+ and Prkcq−/− mice and lysed in ice RIPA buffer (Beyotime) containing protease inhibitor cocktail. Lysates were incubated on ice for 30 min and centrifuged at 12,000 g for 20 min at 4°C. The concentration of proteins was determined using the bicinchoninic acid (BCA) protein assay (Beyotime) according to the manufacturer's instructions. Extracted proteins were subsequently loaded and separated via SDS-PAGE gels and transferred to PVDF membranes. After being blocked with 5% non-fat milk at room temperature for 2 h, the membranes were incubated with primary antibodies and corresponding secondary antibodies. Following by three washes with TBS-Tween-20 (TBST), high-sig ECL western blot analysis substrate (Tanon) was used to detect protein bands. The primary antibodies were used as follows: anti-PRKCQ (13643S, Cell Signaling Technology), anti-GAPDH (AB0038, abways).
4.7 Histology Analysis
Tissues were fixed in modified Davidson's fluid (MDF) for 48 h and dehydrated with a graded ethanol series (70%–100%). After being cleared in xylene and embedded in paraffin, sections were then cut at a 5-μm thick and mounted onto glass slides. These sections were subjected to Periodic Acid-Schiff's (PAS) or H&E reagent for further histological examination routinely.
4.8 Immunofluorescence and TUNEL Assay
For immunofluorescence, paraffin sections were rehydrated and boiled in heat-induced citrate buffer (1.8 mM citric acid, 8.2 mM sodium citrate, pH 6.0) to facilitate antigen retrieval. Samples were then washed with PBS-Triton-X-100 (PBST) and blocked with 5% BSA at room temperature for 2 h. Sections were incubated with primary antibodies and corresponding fluorescence-conjugated secondary antibodies and analyzed using a fluorescence microscope (ZEISS LSM800, Germany). The primary antibodies were used as follows: anti-LIN28A (ab46020, Abcam), anti-γH2AX (ab26350, Abcam), anti-TNP1 (17178-1-AP, Proteintech), anti-SOX9 (A19710, Abclonal), anti-AC-TUBULIN (T6793, Sigma). For TUNEL assay, paraffin sections were performed using TUNEL BrightGreen Apoptosis Detection Kit (Vazyme) according to the manufacturer's instruction.
4.9 Sperm Parameters Analysis
Sperm were released from the cauda epididymis and incubated in fresh human tubal fluid (HTF) media (Irvine Scientific) supplemented with 10% FBS at 37°C for 10 min. A 10 µL sperm suspension was then analyzed for sperm motility test via CASA detection (Hamilton Thorne Inc). Diluted sperm were counted using a hemocytometer for sperm count test.
4.10 Statistical Analysis
Two-tailed unpaired Student's t-test was used to determine the statistical significance and p < 0.05 was considered statistically significant.
Author Contributions
Yu Chen and Hong Chen: data curation, methodology, visualization, writing – original. Zhongyan Han: resources, investigation. Yiqiang Cui, Chenghao Situ, and Yaling Qi: validation. Qing Cheng and Yan Li: conceptualization, funding acquisition, writing – review and editing.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (82371623, 82401873), Science and Technology Project of Changzhou Municipal Health Commission (ZD202327, QN202330), Changzhou Science and Technology Program (CJ20240088), Project of Changzhou Medical Center, Nanjing Medical University (CMCB202324), Science and Technology Development Fund of Nanjing Medical University (NMUB20230057) and Changzhou Key Laboratory of Maternal and Child Health Medicine (Grant No. CM20240013).
Ethics Statement
This study was designed according to the guidelines and regulations of the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University, and approved by the Animal Ethical and Welfare Committee (No. IACUC-1810007-2).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article. The datasets analyzed during this study are available from the corresponding author.




