Low-dose dexamethasone promotes osteoblast viability by activating autophagy via the SGK1/FOXO3a signaling pathway
Abstract
Autophagy contributes to bone homeostasis and development under physiological conditions. Although previous studies have demonstrated the induction of the autophagy machinery by endogenous glucocorticoids (GCs), the precise mechanisms involved have not yet been clarified. The current study aimed to explore the effect of a low dose of GC (10−8 M dexamethasone, Dex) on autophagy in mouse embryonic osteoblastic precursor cells (MC3T3-E1 cells) and the potential mechanisms. The results showed that 10−8 M Dex induced significant time-dependent increases in the expression and activation of serum- and glucocorticoid-induced kinase-1 (SGK1) in MC3T3-E1 cells and that these effects were accompanied by increased cell viability and decreased apoptosis. The autophagy inhibitor 3-MA significantly inhibited Dex-mediated promotion of viability. Moreover, Dex increased LC3II and Beclin-1 levels and decreased SQSTM/p62 levels in a time-dependent manner, and these effects were attenuated by pretreatment with 3-MA. Transfection of Dex-treated MC3T3-E1 cells with shRNA-SGK1 resulted in a significant reduction in cell viability and an increase in apoptosis. 3-MA further exacerbated these effects of SGK1 inhibition. Knocking down SGK1 before Dex exposure significantly reduced the phosphorylated forkhead box O3a (p-FOXO3a)/FOXO3 ratio, suppressed LC3II and Beclin-1 levels, and increased SQSTM/p62 levels in MC3T3-E1 cells, and these effects were amplified by 3-MA. In conclusion, the results revealed that low-dose GC treatment increased osteoblast viability by activating autophagy via the SGK1/FOXO3a pathway.
1 INTRODUCTION
Osteoporosis has emerged as a major global health problem with the increase in the elderly population worldwide. Osteoporosis is a progressive and systemic bone metabolic disease characterized by osteopenia, bone fragility, and increased fracture tendency (J. Liu et al., 2019). Osteoblasts play an important role in bone remodeling by producing extracellular matrix and promoting its mineralization, and abnormal osteoblast function is an important cause of osteoporosis (Lee et al., 2017). Therefore, improving osteoblast viability and function has potential applications in osteogenesis treatments (Al Saedi et al., 2020).
Autophagy is a highly conserved lysosome-dependent degradation pathway for recycling of damaged cytosolic proteins and organelles that facilitates cellular homeostasis and promotes cell survival under stress conditions, such as nutrient deprivation and hypoxia (Kroemer et al., 2010). Induction of autophagy was recently shown to be useful for maintaining bone stability (Pierrefite-Carle et al., 2015; Yin et al., 2019). Endogenous glucocorticoids (GCs) can induce autophagy and contribute to bone metabolism and development under physiological conditions (Jia et al., 2011; T. Wang et al., 2020). Notably, targeting autophagy and homeostasis is potential strategy to protect osteoblast function and slow senescence (Y. Wang et al., 2021). However, the effects of physiological concentrations of GCs on autophagy and t osteoblast protection and the precise mechanisms involved are poorly understood.
Serum- and glucocorticoid-induced kinase-1 (SGK1) is a serine/threonine kinase that was initially identified as an immediate early gene that is transcriptionally regulated by serum and GCs. SGK1 is activated at both the transcriptional and posttranslational levels by a large number of extracellular signals, such as hyperosmotic stress, ultraviolet radiation, heat shock, oxidative stress, and hypoxia (Lang & Cohen, 2001). SGK1 supports cell survival and cell migration, which are prerequisites of tissue repair. Moreover, SGK1 negatively regulates the proapoptotic transcription factor forkhead box O3a (FOXO3a) via phosphorylation and exclusion from the nucleus (Dehner et al., 2008). A previous study has demonstrated that the SGK1/FOXO3a pathway promotes cell survival by inhibiting cell cycle arrest and apoptosis (Brunet et al., 2001). In addition, SGK1 has been shown to act as a switch for autophagy homeostasis, and Xie et al. showed that SGK1 overexpression promoted autophagic flux and enhances the protective effect of hypoxic preconditioning (Xie et al., 2018).
This study examined the effect of low-dose dexamethasone (Dex) on cell viability, autophagy machinery, and the activation of SGK1 in MC3T3-E1 cells. For such purpose, SGK1 was knocked down before Dex exposure, and the phosphorylated FOXO3a (p-FOXO3a)/FOXO3 ratio, cell viability, and the autophagy related proteins expression in MC3T3-E1 cells were evaluated.
2 MATERIALS AND METHODS
2.1 Cell culture and transfection
The murine embryonic osteoblastic precursor cell line MC3T3-E1 was purchased from the Cell Bank of the Chinese Academy of Sciences and cultured in Dulbecco's Modified Eagle's Medium (DMEM)/F12 medium (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), 2 mmol/L glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in a humidified incubator containing 5% CO2. The cells were exposed to 10−8 M Dex, which is commonly used to induce osteoblast differentiation, for different durations. MC3T3-E1 cells maintained in medium without Dex were used as the normal control group. To suppress autophagy, 5 mM 3-methyladenine (3-MA; Sigma-Aldrich) was added to the medium 1 h before each experiment.
The murine expression vectors PGpU6/GFP//Neo-Control and PGpU6/GFP//Neo-SGK1 (SGK1 siRNA) were purchased from or constructed by GenePharma. Briefly, MC3T3-E1 cells were seeded in six-well plates in serum-free DMEM and then treated with a mixture of siRNA and Lipofectamine 3000 (Thermo Fisher Scientific) reagent. After 48 h, the medium was changed, and the cells were incubated with fresh medium for further experiments.
2.2 Cell viability analysis on different time points
Cell viability was analyzed with a cell counting kit-8 (CCK-8; Dojindo) assay according to the manufacturer's instructions. The optical density was measured with a microplate reader (Thermo Fisher Scientific) at 450 nm. The percentage of living cells was calculated based on the ratio of the absorbance of the experimental group to that of the control group (Jiang et al., 2019).
2.3 Flow cytometry analysis of apoptosis
Cells were collected and stained with an Annexin-V/propidium iodide (PI) apoptosis assay kit (Thermo Fisher Scientific) according to the manufacturer's instructions and were analyzed using a FACSCaliburTM flow cytometer (BD Biosciences). Ten thousand cells from each sample were acquired, and the data were analyzed using Cell Quest software (BD Biosciences). Annexin-V+/PI− cells were considered early apoptotic cells.
2.4 Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells (2 × 106) treated with 10−8 M Dex for 30 min, 1, 3, 6, 12, and 24 h using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer's protocol. Aliquots (5 mg) of RNA were reverse-transcribed to form cDNA using a SuperScript™ First-Strand Synthesis System (Thermo Fisher Scientific). The primers for qRT-qPCR were as follows: SGK1 forward: 5ʹ-CACGCCAAACCCTCCGACTTTC-3ʹ, reverse 5ʹ-GCCTTGTGCCTAGCCAGAAGAAC-3ʹ; β-actin forward: 5ʹ-TATGCTCTCCCTCACGCCATCC-3ʹ, reverse 5ʹ-GTCACGCACGATTTCCCTCTCAG-3ʹ. qRT-PCR was conducted according to the manufacturer's instructions (Thermo Fisher Scientific). The reactions were performed on a 7500 Real-Time PCR System (Applied Biosystems). Gene expression was measured with a SYBR Green RT-PCR Kit (Thermo Fisher Scientific), and relative gene expression was determined by normalization to β-actin and the method (Livak & Schmittgen, 2001).
2.5 Western blot analysis
MC3T3-E1 cells were treated, collected, and lysed with RIPA buffer supplemented with complete protease inhibitor cocktail tablets. The protein concentration was measured using a DC Protein Assay Kit (Bio–Rad Laboratories). Equal amounts of protein were separated on SDS-polyacrylamide gels and transferred onto PVDF membranes (Millipore). The membranes were then incubated overnight at 4°C with SGK1 (D27C11) (catalog #12103), phospho-SGK1 (Ser78) (D36D11) (catalog #5599), FoxO3a (75D8) (catalog #2497), Beclin-1 (D40C5) rabbit monoclonal antibody (catalog #3495), Phospho-FoxO3a (Ser253) rabbit polyclonal antibody (catalog #9466) (1:1000; Cell Signaling), a LC3 rabbit polyclonal antibody (catalog #NB100-2220) (1:500; Novus), a SQSTM1/p62 (catalog #ab56416) (1:1000; Abcam), and a β-actin (clone AC-15) monoclonal antibody (catalog #ab56416) (1:5000; Sigma-Aldrich). Next, the membranes were blotted with HRP-conjugated anti-rabbit or anti-mouse IgG (1:5000, Jackson). The protein bands were visualized using Pierce™ ECL Plus Western blot analysis substrate (catalog #32132) according to the manufacturer's instructions and developed with film. The relative density was quantified using ImageJ software (Java; NIH) (Schneider et al., 2012).
2.6 Statistical analysis
All experiments were conducted in triplicate, and all data were presented as the mean ± standard deviation. Statistical analysis was conducted using the GraphPad Prism software (GraphPad Prism 9.0 for windows). Statistical differences in multiple groups were determined by multiple comparisons with ANOVA followed by Tukey post hoc tests. Statistical differences between two groups were determined by two-tailed unpaired or paired Student t-test. A normal distribution of the data was shown using the Shapiro−Wilk test. A value of p < .05 was considered to indicate statistical significance.
3 RESULTS
3.1 Autophagy is dynamically involved in the effect of dexamethasone on MC3T3-E1 cell viability
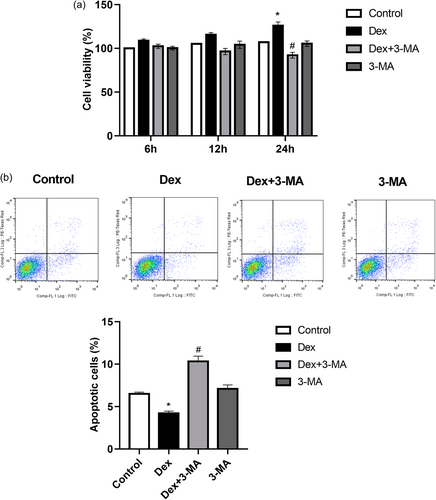
To clarify the effect of exposure to low-dose Dex for different durations on MC3T3-E1 cell viability and to determine whether the effect was associated with autophagy, MC3T3-E1 cells were pretreated with the autophagy inhibitor 3-MA before being incubated with Dex. The results showed that the viability of MC3T3-E1 cells treated with a low dose of Dex (10−8 M) was significantly increased at 24 h than that of the control cells (Figure 1a). 3-MA significantly inhibited the Dex-mediated promotion of MC3T3-E1 cell viability (Figure 1a). Moreover, flow cytometry showed that the decrease in the proportion of apoptotic cells induced by low-dose Dex (10−8 M) was inhibited by 3-MA (Figure 1b). Therefore, it was hypothesized that the effect of low-dose Dex on MC3T3-E1 cell viability may be closely related to autophagy.
3.2 Dexamethasone dynamically induces SGK1 expression and activation in MC3T3-E1 cells
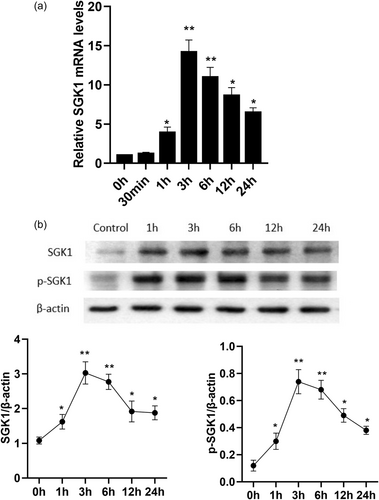
The effect of low-dose Dex exposure for different durations, on SGK1 gene/protein expression are shown in Figure 2a,b. Under normal circumstances, SGK1 was weakly expressed in MC3T3-E1 cells. Treating MC3T3-E1 cells with 10−8 M Dex induced a significant time-dependent increase in the expression of SGK1 at both the mRNA (Figure 2a) and protein levels compared to that in the control group (Figure 2b). Interestingly, the low-dose Dex-mediated induction of SGK1 expression and phosphorylation in MC3T3-E1 cells were very fast; the levels began to increase after 1 h of Dex stimulation, peaked at 3 h, and then decreased gradually until after 24 h of Dex exposure (Figure 2). These results demonstrate that low-dose Dex exposure promotes the expression and activation of SGK1 in MC3T3-E1 cells.
3.3 Dexamethasone induces time-dependent activation of autophagy in MC3T3-E1 cells
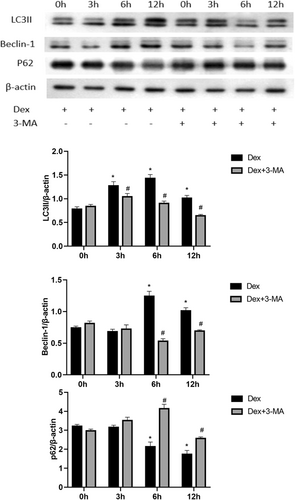
The primary method for measuring autophagic activity involves the analysis of Beclin-1, SQSTM1/p62, and LC3I-to-LC3II processing. Exposure to low-dose Dex (10−8 M) for different durations led to time-dependent changes in autophagy in MC3T3-E1 cells, which peaked at 6 h, as indicated by the expression of LC3II and Beclin-1. However, the protein levels of the selective autophagy substrate SQSTM/p62 were depleted after 6 h of Dex stimulation (Figure 3). Compared to those in the Dex-treated group, 3-MA-pretreated cells showed significant time-dependent decreases in the expression of LC3II and Beclin-1 and an increase in the level of SQSTM/p62 (Figure 3).
3.4 SGK1 promotes viability and inhibits apoptosis in MC3T3-E1 cells exposed to dexamethasone
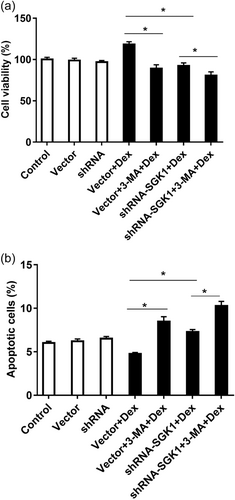
To further study the effects of low-dose Dex (10−8 M)-induced SGK1 expression and phosphorylation, SGK1 expression was knocked down with shRNA, and autophagy was pharmacologically inhibited with 3-MA. MC3T3-E1 cells transfected with the empty vector or SGK1 shRNA (shRNA-SGK1) for 48 h were incubated with 3-MA for 1 h and then treated with Dex for 24 h. After these treatments, cell viability and apoptosis were examined. MC3T3-E1 cells transfected with shRNA-SGK1 exhibited significant lower cell viability (Figure 4a) and greater apoptosisthan vector-transfected cells cultured with Dex (Figure 4b). Moreover, Dex-induced MC3T3-E1 cell survival was effectively attenuated by the autophagy inhibitor 3-MA (Figures 4a), and 3-MA further exacerbated the effects of SGK1 inhibition on cell viability and apoptosis (Figures 4a,b).
3.5 SGK1 promotes autophagy in dexamethasone-treated MC3T3-E1 cells via FOXO3a
To further explore the mechanism of Dex-induced autophagy, the phosphorylation levels of FOXO3a, and autophagy-associated proteins after shRNA-SGK1 transfection with or without 3-MA treatment were measured. The results revealed that pretreatment with 3-MA alone before Dex incubation notably decreased the expression of p-FOXO3a and the autophagy-associated proteins LC3II and Beclin-1 and distinctly increased the expression of SQSTM/p62 (Figure 5).
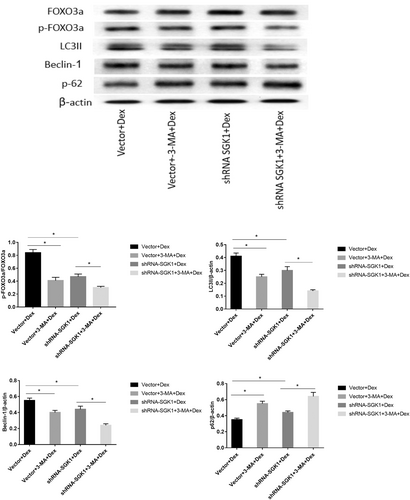
Furthermore, SGK1 inhibition significantly decreased FOXO3a phosphorylation and LC3II and Beclin-1 levels and promoted of SQSTM/p62 expression in response to Dex. 3-MA further reduced the levels of p-FOXO3a, LC3II, and Beclin-1 and increased the level of SQSTM/p62 in the presence of Dex and SGK1 inhibition (Figure 5). These findings indicate that in response to low-dose Dex exposure, SGK1-mediated autophagy may contribute to cell survival via phosphorylation and inactivation of FOXO3a.
4 DISCUSSION
The current study demonstrated that SGK1 was upregulated and activated in MC3T3-E1 cells incubated with a low dose of Dex (10−8 M), an inducer of osteoblast differentiation. SGK1 knockdown attenuated the Dex-induced effects on cell viability, autophagy levels and the p-FOXO3/FOXO3a ratio, confirming the protective effect of SGK1 on osteoblast differentiation. In addition, the findings showed that inhibiting autophagy exacerbated the damaging effects of SGK1 knockdown after low-dose Dex exposure, suggesting that SGK1 might protect osteoblast survival during the differentiation process by promoting autophagy and the FOXO3a signaling pathway.
Dex is a commonly administered anti-inflammatory and immunosuppressive drug that is used in clinical practice to treat a variety of diseases. GC-induced osteoporosis (GIO) is considered to be the most common form of secondary osteoporosis due to the widespread use of GCs. Interestingly, endogenous GCs are essential for the maintenance of bone homeostasis, and excess GCs may impair bone formation or facilitate bone resorption (Hartmann et al., 2016). Therefore, it is necessary to uncover the mechanism by which GCs maintain the balance of bone homeostasis under physiological conditions and further develop effective strategies for treating or preventing GIO.
Bone homeostasis is a dynamic balance mediated by osteoblasts, osteoclasts, bone marrow mesenchymal stem cells, and osteocytes. Recent studies have demonstrated that different doses of GCs lead to different bone cell fates (Shi et al., 2015; Y. Wang et al., 2021). Osteoblasts, which are derived from mesenchymal stem cells, are mainly responsible for bone formation and development. Zhang et al. (2018) found that a high dose of Dex (≥10−6 M) accelerates osteoblast apoptosis, while a low-dose of Dex (10−8 M) increases osteoblast viability in the early stage. This finding is consistent with the experimental results of the current study. However, the underlying mechanism is still complex and requires further investigation.
Autophagy is a highly conserved catabolic process that is essential for cell growth, survival, differentiation, development, and homeostasis (Dikic & Elazar, 2018). It has been shown that autophagy plays a critical role in physiological processes and numerous pathological conditions, including inflammation, neurodegeneration, cancer, and bone metabolic diseases (Shapiro et al., 2014). Under physiological conditions, autophagy is responsible for the removal of damaged or excessive organelles, whereas under pathological conditions, autophagy aids in the redistribution of intracellular nutrients to meet the nutrient and energy requirements for survival. Although an appropriate autophagy level is a prerequisite for maintenance of cell homeostasis and survival, excessive activation of autophagy generally leads to programmed cell death; in this way, autophagy acts as a double-edged sword (Shintani & Klionsky, 2004). Over the past decade, many studies have suggested that autophagy in osteoblasts plays critical roles in bone homeostasis and GIO (Nollet et al., 2014). The results demonstrated that 10−8 M Dex induced osteoblast autophagy and maintained osteoblast viability. The positive effect of 10−8 M Dex on the occurrence of autophagy was confirmed, as reflected by increased expression of beclin-1 and LC3II and decreased expression of SQSTM/p62. Furthermore, this study showed that 10−8 M Dex increased LC3II and Beclin-1 levels and decreased SQSTM/p62 levels in a time-dependent manner, and that these effects were attenuated by pretreatment with the autophagy inhibitor 3-MA. Moreover, 3-MA significantly inhibited Dex-mediated promotion of cell viability. Mice with essential autophagy gene knockout and osteoblast-specific autophagy have previously been used to demonstrate that autophagy deficiency increases oxidative stress and reduces mineralization capacity, showing that autophagy in osteoblasts is involved in bone homeostasis (L. Wang et al., 2019). However, the precise signaling pathway by which autophagy affects the viability of osteoblasts exposed to low doses of GCs has rarely been reported.
SGK1, which is related to Akt (also called PKB), is a serine/threonine kinase that can be activated by PI3K and is been known for its role in ion channel modulation and cell survival in response to stress stimuli. SGK1 participates in the regulation of transport, hormone release, tumor growth, neurodegeneration, cell proliferation, and apoptosis in multiple cell lines (Lang et al., 2006; Leong et al., 2003). In vitro studies have indicated that SGK1 protein levels and activation are significantly stimulated by H2O2 exposure. In human proximal tubular cells, SGK1 inhibition increases the sensitivity to H2O2-induced oxidative stress injury from the under control condition, as indicated by increases in apoptotic cell death and mitochondrial dysfunction (Jiang et al., 2019). Li et al. (2018) reported that SGK1 overexpression significantly attenuates A549 cell apoptosis and reduces the reactive oxygen species generation induced by particulate matter (PM2.5). The present study showed that treating MC3T3-E1 cells with 10−8 M Dex induced a significant time-dependent increase in the expression of SGK1 at the mRNA and protein levels; and the levels began to increase after 1 h of Dex stimulation and peaked at 3 h. Knocking down SGK1 weakened the Dex-mediated promotion of cell viability.
In addition to its aforementioned functions, SGK1 is known to act as a switch in autophagy homeostasis. However, there is controversy regarding the effect of SGK1 on autophagy in different cell lines (W. Liu et al., 2017; Maestro et al., 2020). Notably, the results of this study showed that the effect of SGK1 on autophagy paralleled the significant increase in the viability of MC3T3-E1 cells exposed to a low dose of Dex. Moreover, inhibiting SGK1 significantly decreased the expression of the autophagy markers LC3II and Beclin-1, promoted the expression of SQSTM/p62 and increased apoptosis in Dex-treated MC3T3-E1 cells compared to control cells. 3-MA further exacerbated the effects of SGK1 inhibition on cell viability and apoptosis.
FOXO3a, which is a member of the forkhead transcription factor family, is a downstream target of SGK1. SGK1 phosphorylates FOXO3a, leading to FOXO3a translocation from the nucleus to the cytoplasm and to inhibition of FOXO3a-dependent transcription, thereby preventing FOXO3a from inducing apoptosis and/or cell cycle arrest (Brunet et al., 2001). FOXO3a has been reported to actively promote apoptosis by inducing the expression of Bcl-2 family members (Fu & Tindall, 2008). Similarly, Chen et al revealed that SGK1 activation significantly increases FOXO3a phosphorylation and inhibited the subsequent expression of Bcl-2-interacting mediator of cell death (Bim). SGK1 mediated the hypotonic protective effect against H2O2-induced apoptosis in rat basilar artery smooth muscle cells by inhibiting the FOXO3a/Bim signaling pathway (Chen et al., 2020). In addition, recent studies have indicated that FOXO3a overexpression not only decreased HIF-1α protein levels but also inhibited HIF-1α transcriptional activity, as evidenced by decreased expression of the HIF-1 target genes EPO, HO-1, and Bnip3, which are known to regulate autophagy (Regmi et al., 2020; Xie et al., 2018). The current study showed that knocking down SGK1 before Dex exposure significantly decreased the phosphorylated p-FOXO3a/FOXO3 ratio, suppressed LC3II and Beclin-1 levels, and increased SQSTM/p62 levels in MC3T3-E1 cells, and these effects were amplified by 3-MA. These results reveal that low-dose of GC treatment increases osteoblast viability by activating autophagy via the SGK1/FOXO3a pathway.
5 CONCLUSION
The findings of the current study have indicated that a low-dose of Dex could upregulate SGK1 expression, appropriately activate autophagy and promote cell survival by dephosphorylating and inactivating FOXO3a in MC3T3-E1 cells. This study thus provides new insight into autophagic regulatory mechanisms in the early stage of low-dose Dex-mediated osteoblast differentiation and identifies novel therapeutic strategies to improve osteoblast functions. Future in vivo studies with genetic models will be required to clarify the role of SGK1 in osteoblast differentiation and the mechanism of SGK1-mediated autophagy. SGK1 has emerged as a novel therapeutic strategy that is likely to attract considerable research attention in the coming years.
AUTHOR CONTRIBUTIONS
Xiang-Hua Yu designed and performed the experiments. Xiang-Hua Yu contributed to the cell culture and western blot analysis. Sheng-Xiang Zhang performed qRT-PCR and cell viability analysis. Xiao-Ming Xu was responsible for the statistical analysis. Xiang-Hua Yu wrote the paper. All authors have read and approved the final version of the manuscript submitted for publication.
ACKNOWLEDGMENT
This work was supported by grants from the Minhang District Science and Technology Committee, Shanghai (No. 2021MHZ045).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed in the current study were included in the published article and any further explanations are available from the corresponding author upon reasonable request.




