GPRC6A is a key mediator of palmitic acid regulation of lipid synthesis in bovine mammary epithelial cells
Abstract
Fatty acids (FAs) can promote lipid synthesis in the mammary gland via stimulating lipogenic gene expression, but the underlying molecular mechanism is still not fully understood. Here, we showed the dose-dependent effects of palmitic acid (PA) on lipid synthesis in primary bovine mammary epithelial cells (BMECs) and explored the corresponding molecular mechanism. BMECs were treated with PA (0, 50, 100, 150, and 200 μM), and the 100 μM treatment had the best stimulatory effect on lipid synthesis and expression and maturation of sterol regulatory element-binding protein 1c (SREBP-1c) in cells. Inhibition of phosphatidylinositol 3-kinase (PI3K) almost totally blocked the stimulation of PA on SREBP-1c expression, whereas protein kinase Cα (PKCα) knockdown only partially decreased the stimulation of PA on SREBP-1c expression but abolished the stimulation of PA on its maturation. Knockdown of GPR120 did not change the stimulation of PA on the SREBP-1c signaling. G protein-coupled receptor family C group 6 member A (GPRC6A) knockdown almost totally blocked the stimulation of FA on PI3K and PKCα phosphorylation as well as SREBP-1c expression and maturation. Furthermore, PA dose-dependently promoted GPRC6A expression and plasma membrane localization. Together, these above results reveal that GPRC6A is a key mediator of PA signaling to lipid synthesis in BMECs via the PI3K/PKCα–SREBP-1c pathways.
1 INTRODUCTION
Milk fat, including triglycerides (TGs) (∼98%) and other lipid components, provides both critical energy and essential nutrients to the growth of the newborn. Fatty acids (FAs) are the substrates of milk fat synthesis in the mammary gland, which come from blood circulation and de novo synthesis from acetate (in ruminants), glucose, and other dietary precursors. For lactating mammals, palmitic acid (PA) is one of the most abundant FAs in milk (Lv et al., 2018). In mammary epithelial cells (MECs), the lipid is synthesized from FAs and other precursors by a series of enzymes whose expression is controlled by the transcription factor sterol regulatory element-binding protein 1c (SREBP-1c) (Xu et al., 2016).
SREBP-1c is primarily expressed as a premature form (full length of SREBP-1c, fSREBP-1c) in the cytoplasm, attached to the endoplasmic reticulum membrane. When activated, fSREBP-1c is cleaved into an N-terminal domain (nuclear SREBP-1c, nSREBP-1c), then nSREBP-1c translocates to the nucleus and binds to the sterol regulatory element DNA sequence TCACNCCAC, thus upregulating the synthesis of lipogenic genes (Han et al., 2018; N. Li et al., 2014).
SREBP-1c is a major regulator of milk fat synthesis in MECs (N. Li et al., 2014; P. Li et al., 2019), and hormones and amino acids can stimulate SREBP-1c expression and maturation via the phosphatidylinositol 3-kinase (PI3K) signaling (M. Zhang et al., 2018). G protein-coupled receptor 120 (GPR120) can mediate the signaling of long-chain FAs to lipid synthesis via the PI3K pathway in some tissues (Katsuma et al., 2005; Meng et al., 2017; M. Zhang & Qiu, 2019), but whether GPR120 plays a key role in MECs still needs further research. FAs can upregulate or downregulate SREBP-1c expression and activation (P. Li et al., 2018; Lv et al., 2018; Shi et al., 2017), and GPCR can mediate FA signaling to SREBP-1c still need to be determined.
Protein kinase C (PKC), a family of serine/threonine protein kinase enzymes downstream of GPCRs, plays an important role in cell growth, differentiation, and cell metabolism. PKC subclasses in mammalian tissues are divided into three groups: A, B, and C. Group A is called classical PKC, including α, β I, β II, and γ subclasses. Group B is a new type of PKC, including δ, ε, η (L), and θ subclasses. Group C is atypical PKC (aPKC), which is composed of ζ and λ subclasses. Several previous reports provide evidence that PKC might affect the SREBP-1c signaling. Inhibition of hepatic aPKC diminished the expression of SREBP-1c in human hepatocytes (Sajan et al., 2018). PKCβ increased SREBP-2 nuclear translocation in hepatic cells (Wong et al., 2016). A PKC substrate myristoylated alanine-rich C-kinase substrate negatively regulated lipid accumulation in the liver (Song et al., 2018). These reports suggest that PKC is involved in the regulation of lipid synthesis.
FAs can function via PKC activation. A pan-PKC inhibitor prevented the PA-induced expression of endothelin-1 in human aortic endothelial cells (J. Zhang et al., 2018). A short period of treatment with PA reduced the cluster of differentiation 36 (CD36) and increased glucose transporter type 4 pathway protein levels in cardiomyoblast cells, and these effects were reversed by knockdown of PKCζ (Chen et al., 2016). In ruminants, PA consistently increases the expression of CD36 in bovine mammary epithelial cells (BMECs), suggesting a different regulatory mechanism of PA in ruminants (Bionaz et al., 2012). PKCα mediated PA-induced autophagy in human gastric cancer cells (Jia et al., 2016). PKCδ inhibition lessened FAs-induced apoptosis of INS-1β cells (Qin et al., 2014). It has not been reported whether PKC mediates the stimulation of FAs on the SREBP-1c signaling.
G protein-coupled receptor family C group 6 member A (GPRC6A) is a widely expressed master regulator of energy metabolism and endocrine networks. GPRC6A serves as a sensor and mediator of multiple ligands, including basic amino acids, osteocalcin, testosterone, other steroids, and various cations (Pi et al., 2016). Previous reports have shown that GPRC6A can function through the ERK1/2, PI3K/protein kinase B (AKT), mammalian target of rapamycin/S6K1, and SREBP signaling pathways (Jørgensen & Bräuner-Osborne, 2020; X. Li et al., 2019; Ye et al., 2019). It is still unknown whether GPRC6A participates in the regulation of FA on lipid synthesis.
It is still not known well the molecular mechanism of FA signaling to SREBP-1c expression and maturation for lipid synthesis in BMECs. We previously found that GPRC6A might be associated with FA signaling to the SREBP-1c pathway (X. Li et al., 2019). In this study, we aim to determine the dose-dependent effect of PA on the SREBP-1c pathway and lipid synthesis in BMECs and explore the mediatory molecular mechanism of GPRC6A in this regulation.
2 MATERIALS AND METHODS
2.1 Isolation, culture, and identification of primary cells
Primary BMECs were separated from mammary gland tissue of a Chinese Holstein dairy cow in the mid-lactation period. All processing steps involving cows were performed according to the Animal Experiment Guide of Northeast Agricultural University. Small pieces of lactating mammary gland tissues were taken, carefully washed with 0.9% sterile saline, then soaked in 75% ethanol for 5 min, washed three times with d-Hank's solution, and next washed three times with fluconazole (0.30 μg/ml). Then, the tissues were cut into pieces of about 1 mm3 in size. The pieces were inoculated into cell culture flasks covered with rat tail collagen in a 5% CO2 incubator at 37°C, and after 4 h, a cell culture medium (Dulbecco's modified Eagle's medium/F12 containing 15% fetal bovine serum, 10X penicillin/streptomycin, 5X amphotericin) was added to the culture flasks. After the tissue pieces were cultured for 7 days, the cells crawling out of the tissue pieces were treated by trypsin digestion, and purified BMECs were obtained after 4–5 passages.
The purity of BMECs was identified by morphological observation and immunofluorescence observation of the subcellular localization of cytokeratin 18 (CK18). Briefly, BMECs were passed into a six-well plate with a coverslip, and cultured to 50%–70% confluence. Then, the coverslip was covered with 4% formaldehyde for 15 min, and the next cells on the coverslip were incubated at 37°C with 5% bovine serum albumin plus 0.1% Triton X-100 in TBS for 1.5 h. Next, cells were incubated with the primary antibody to CK18 (sc-51582; Santa Cruz) at 4°C overnight. Cells were further incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (A0562; Beyotime) in the dark room at 37°C for 1 h. Finally, cell nuclei were dyed with 4′,6-diamidino-2-phenylindole (DAPI) in the dark room for 10 min. Cell images were observed under a confocal microscope (TCS-SP2 AOBS; Leica).
2.2 Cell treatments
To eliminate the interference with the composition of the culture medium, cells were transferred into serum-free OPTI-MEM I medium and cultured for 12 h before experimental assays. To detect the effects of PA, different concentrations of PA (p815432; Macklin) (0, 50, 100, 150, and 200 μM dissolved in absolute ethanol) were added to the medium. To specifically inhibit PI3K activity, LY294002 (15 μM) was added to the medium. Cells were treated for 24 h and collected for testing.
2.3 Determination of the amount of TGs in the culture medium
The amount of TGs in the culture medium was determined using a TG Detection Kit (E1013; Applygen). Briefly, 10 μl of cell supernatant and 190 μl of the mixed working solution were mixed into a 200 μl centrifuge tube, then incubated at 37°C for 10 min. The optical density value of the product at 550 nm was measured by a microplate reader. The standard curve was also determined according to the manufacturer's instructions, and the amount of TGs was then calculated.
2.4 Detection of lipid droplets
Cells were passed into a six-well plate covered with a coverslip, then cultured to 30%–50% confluence. After treatments, cells on the coverslip were blocked with 4% formaldehyde for 20 min, then washed with PBS (pH 7.2) three times, and next lipid droplets (LDs) in cells were dyed with a hydrophobic fluorescent probe Bodipy 493/503 (Invitrogen, Carlsbad, CA, USA) (1 μg/mL) in the dark for 15 min. Finally, nuclei were treated with DAPI for 10 min in the dark, then cells were washed 3 times with PBST containing 0.1% Tween-20. The cells on the coverslips were observed with a laser confocal microscope (TCS-SP2 AOBS, Leica). The area-integrated optical density (AIOD) of LDs points per cell was analyzed by using ImageJ.
2.5 Cell number counting
The number of live cells was automatically monitored by using a cell counter (Model DT CASY cell counter; Scharfe System GmbH), according to the manufacturer's protocol.
2.6 Western blotting
BMECs were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime). Cell lysates were collected and heated at 100°C for 3 min. Protein concentration was measured using a BCA Protein Assay Kit (Beyotime). Protein separation was performed on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (20 μg/lane), then proteins were transferred from the gel to a nitrocellulose membrane. The membrane was then sealed with 5% skim milk at 37°C for 1.5 h, and then incubated with the primary antibody at 4°C overnight. The primary antibodies were as follows: GPRC6A (ab128661), Abcam; SREBP-1c (14088-1-AP) and PKCα (21991-1-AP), Proteintech; nSREBP-1c (bs-1402R), PI3K (p110α) (bs-2067R), phospho-PI3K (p110α (Tyr317)) (bs-5570R) and p-PKCα (Thr638) (bs-3333R), Bioss; and phospho-AKT1 (Thr308) (sc-135650), AKT1 (sc-1618), and β-actin (sc-47778), Santa Cruz. After overnight treatment, the membrane was incubated with the horseradish peroxidase-labeled goat anti-rabbit secondary antibody (A0208; Beyotime) at 37°C for 1 h, and then washed three times with TBST. Finally, the protein bands were detected by enhanced chemiluminescence reagents (Applygen) and analyzed by ImageJ software. β-actin served as an internal reference.
2.7 Small interfering RNA transfection
Three small interfering RNAs (siRNAs) targeting different coding sequences of the GPRC6A or PKCα messenger RNA (mRNA) were designed and purchased from a company (GenePharma). When the cells in the six-well plate reached 60% confluence, each siRNA was mixed with the transfection reagent siRNA-Mate (GenePharma), then transferred into the cells for 24 h per the manufacturer's protocol. The knockdown efficiency was validated by Western blotting analysis of the protein level of GPRC6A or PKCα in cells transfected with one of the three siRNAs, and the one having the best inhibitory effect was selected for further use. The siRNA sequences of GPRC6A selected were as follows: sense 5′-GGAAUACAGUUCUGGGCATT-3′, antisense 5′-UUGCCCAGAACUGUAUUCCTT-3′. The siRNA sequences of PKCα were as follows: sense 5′-GCUGCUGUACGAGAUGCUATT-3′, antisense 5′-UAGCAUCUCGUACAGCAGCTT-3′.
2.8 Immunofluorescence staining
Cells were treated as the approach aforementioned for the identification of cell purity. The primary antibody to GPRC6A (ab90677; Abcam) was then added, and cells were incubated at 4°C overnight. After washing three times with PBST, cells were further incubated with FITC-conjugated secondary antibody at 37°C for 1 h under dark conditions. Finally, the nuclei were stained with DAPI at room temperature for 10 min in the dark. GPRC6A expression in cells was then observed with a laser confocal microscope, and the AIOD of GPRC6A per cell was analyzed using ImageJ.
2.9 Statistical analysis
Each experiment consisted of three or five sets of technical replicates, and the data are expressed as mean ± standard error. The concentrations of PA were obtained through multiple rounds of screening, to show the optimal PA concentration on lipid synthesis. The difference among the means was analyzed using the analysis of variance followed by Tukey's post hoc test. All data were considered statistically significant at p < .05.
3 RESULTS
3.1 PA dose-dependently promotes milk fat synthesis in BMECs
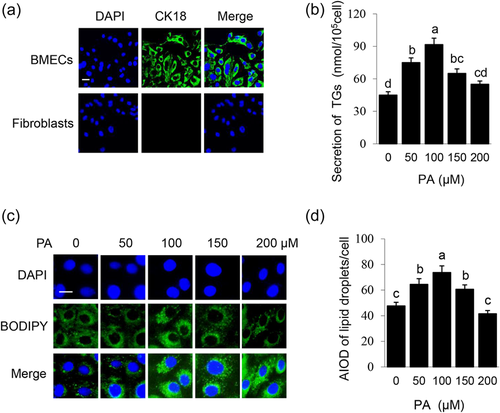
The purity of primary BMECs was identified by morphological and immunofluorescence observation. The purified BMECs were oval or pebble-shaped and grew an island shape. When BMECs and fibroblasts were mixed, they grew in different regions. Purified BMECs exhibited positive CK18 expression which was negative in fibroblasts (Figure 1a). BMECs were treated with different concentrations of PA (0, 50, 100, 150, and 200 μM) for 24 h. With the increase of PA concentration, TGs secreted by cells (Figure 1b) and LDs formation in cells (Figure 1c,d) were both increased, peaked at 100 μM, then gradually decreased. These data showed that PA promotes lipid synthesis in BMECs in a dose-dependent manner.
3.2 PA dose-dependently stimulates signaling pathways related to milk fat synthesis
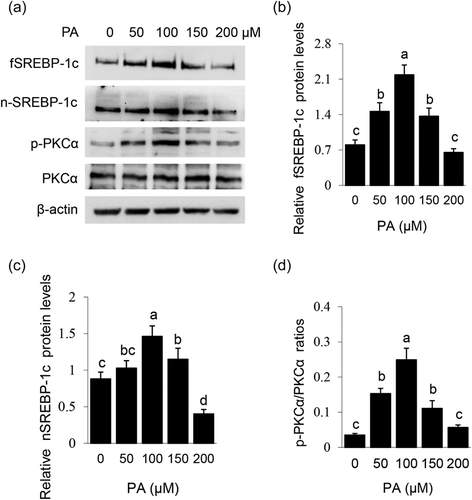
Effects of PA on the signaling pathways related to lipid synthesis were also detected. PA dose-dependently stimulated protein levels of fSREBP-1c (Figure 2a,b) and nSREBP-1c (Figure 2a,c), and PKCα phosphorylation (Figure 2a,d). These data reveal that PA stimulates SREBP-1c expression and maturation, and also suggest that PKCα might be involved in PA-stimulated lipid synthesis.
3.3 PI3K mediates the stimulation of PA on SREBP-1c expression
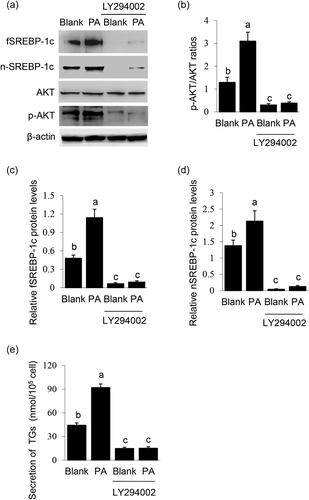
The effects of PI3K inhibition on PA-stimulated SREBP-1c expression and maturation were observed. LY294002 treatment led to a significant decrease in AKT phosphorylation (Figure 3a,b), showing its effective inhibition on PI3K activation. PI3K inhibition totally blocked PA-stimulated protein levels of fSREBP-1c (Figure 3a,c) and nSREBP-1c (Figure 3a,d) and TGs secretion by cells (Figure 3e). These data demonstrate that PI3K is a key mediator of the induction of PA on SREBP-1c expression and subsequent maturation.
3.4 PKCα mediates the stimulation of PA on SREBP-1c maturation
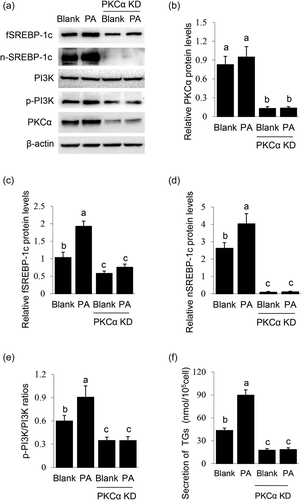
Previous reports have shown that members of the PKC family can affect lipid synthesis and SREBP-1c signaling. Furthermore, PKCα is widely expressed and plays an important regulatory role in MECs (Cui et al., 2019; Llorens et al., 2019; Pham et al., 2017). We thus determined the effects of PKCα knockdown on PA-induced SREBP-1c expression and maturation. PKCα knockdown (Figure 4a,b) only partially decreased the stimulation of PA on fSREBP-1c protein level (Figure 4a,c), but almost totally abolished the stimulation of PA on nSREBP-1c protein level (Figure 4a,d) and TGs secretion by cells (Figure 4f). Furthermore, PKCα knockdown only partially decreased the stimulation of PA on PI3K activation (Figure 4a,e), suggesting the weak effect of PKCα on the PI3K pathway and SREBP-1c expression. Together, these above data suggest that PKCα mainly mediates the stimulation of PA on SREBP-1c maturation.
3.5 GPR120 does not affect PA signaling to SREBP-1c expression and maturation
GPR120 is acknowledged as a GPCR that senses long-chain FAs and mediates their signaling to lipid synthesis. We speculated that GPR120 might play a role in PA-stimulated SREBP-1c expression and maturation in BMECs. Unexpectedly, PA did not affect GPR120 protein level in BMECs (Figure 5a,b). Furthermore, GPR120 knockdown (Figure 5c,d) did not affect PA-stimulated protein levels of fSREBP-1c (Figure 5c,e) and nSREBP-1c (Figure 5c,f). These data suggest that GPR120 might not participate in PA signaling to SREBP-1c expression and maturation in BMECs.
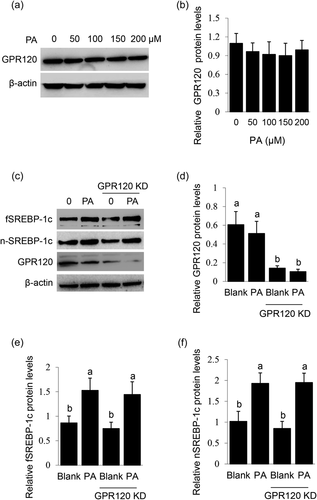
3.6 GPRC6A mediates the stimulation of PA on the SREBP-1c signaling
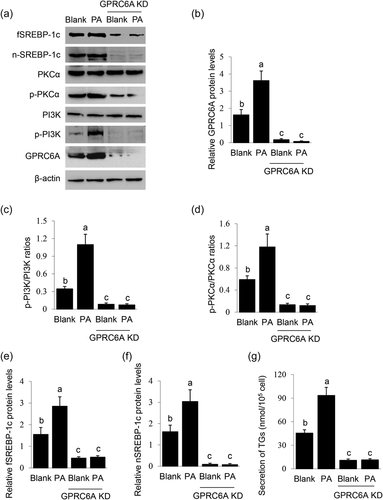
We previously found that FAs induced GPRC6A expression in BMECs (X. Li et al., 2019), and this evoked us to determine whether GPRC6A is involved in PA signaling to SREBP-1c. GPRC6A knockdown (Figure 6a,b) almost totally blocked the stimulation of PA on PI3K activation (Figure 6a,c, and PKCα activation (Figure 6a,d). GPRC6A knockdown also significantly decreased PA-stimulated protein levels of fSREBP-1c (Figure 6a,e) and nSREBP-1c (Figure 6a,f) and TGs secretion by cells (Figure 6g). These data demonstrate that GPRC6A is a key mediator of the stimulation of PA on the PI3K/PKCα-SREBP-1c signaling.
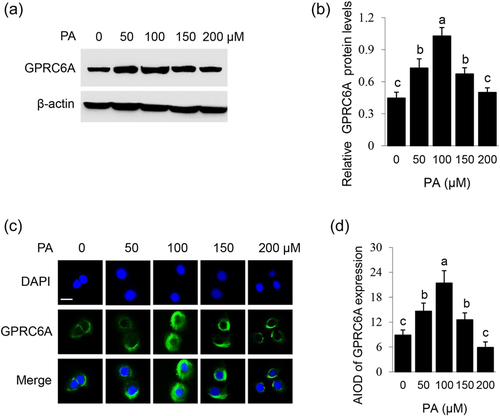
3.7 PA dose-dependently promotes GPRC6A expression and plasma membrane localization
To show whether PA can positively regulate the GPRC6A signaling, effects of PA on GPRC6A expression and subcellular localization were further observed. PA dose-dependently affected the protein level of GPRC6A in BMECs, with the most stimulatory effect at 100 μM (Figure 7a,b). Immunofluorescence observation detected that PA stimulated plasma membrane localization of GPRC6A, and the stimulatory effect also peaked at 100 μM (Figure 7c,d). These data demonstrate that PA promotes GPRC6A expression and plasma membrane localization in a dose-dependent manner.
4 DISCUSSION
We previously found that GPRC6A might be involved in FAs signaling to lipid synthesis, but the role and molecular mechanism of GPRC6A in this process have not been uncovered yet. In this study, we show that PA dose-dependently promotes secretion of TGs, formation of LDs in cells, and SREBP-1c expression and maturation. PI3K is required for PA to stimulate SREBP-1c expression, whereas PKCα is mainly needed for PA to stimulate SREBP-1c maturation. GPR120 is not a mediator of the stimulation of PA on the SREBP-1c signaling, whereas GPRC6A is required for PA to trigger PI3K and PKCα activation and subsequent SREBP-1c expression and maturation. PA dose-dependently promotes GPRC6A expression and plasma membrane localization.
Our experimental data showed that PA dose-dependently promoted lipid synthesis, SREBP-1c expression and maturation, PKCα phosphorylation, and GPRC6A expression, and plasma membrane localization. These data elucidate that, at low concentrations, PA is a key stimulator on cellular signaling pathways leading to lipid synthesis, whereas at higher concentrations, PA inhibits these signaling pathways and might have cytotoxic effects. Previous reports show that an overload of PA has a lipotoxicity impact on cell function and induces cell apoptosis (Chu et al., 2019; Deng et al., 2020; H. Zhang et al., 2021). It is worth studying in the future the dose-dependent effect of PA on apoptosis of BMECs.
Our data showed that PI3K knockdown blocked the signaling of PA and GPRC6A to SREBP-1c expression and subsequent maturation. PI3K is a key regulator for extracellular stimuli such as hormones and amino acids to promote milk fat synthesis in MECs. Methionine and estrogen stimulate the nuclear factor of κB1 activation for SREBP-1c gene expression and milk fat synthesis in BMECs in a PI3K-dependent manner (X. Huang et al., 2017). Taurine stimulates SREBP-1c gene expression and milk fat synthesis via the GPR87–PI3K–SETD1A signaling (Yu et al., 2019). PI3K is a key mediator of PA-regulated cell signaling pathways and physiology (F. Huang et al., 2019; Zhou et al., 2019). PA inhibits the proliferation and metastasis of prostate cancer cells via decreasing PI3K activation (Zhu et al., 2021). Increasing evidence show that PI3K is a downstream effector of GPRC6A to regulate cell proliferation and lipid synthesis under the stimulation of basic amino acids (Fujiwara et al., 2014; P. Li et al., 2019). From our experimental data and these previous reports, we conclude that PI3K is a key mediator of PA-induced GPRC6A-mediated SREBP-1c expression and lipid synthesis.
The experimental data showed that PKCα knockdown abrogated PA-induced SREBP-1c maturation, but only partially decreased PI3K and SREBP-1c expression. To date, it is unknown how PKCα regulates SREBP-1c maturation. A previous study has revealed that 3,5-diiodo-l-thyronine treatment of HepG2 cells blocks the proteolytic cleavage of SREBP-1, without affecting its gene expression, which process is dependent on PKC-δ activation (Rochira et al., 2013). PKCβ increases SREBP-2 nuclear translocation but do not induce its mRNA expression (Wong et al., 2016). These findings firmly support our experimental data that PKCα mainly controls PA-stimulated SREBP-1c maturation. The observation that PKCα knockdown can weakly decrease PI3K activation and SREBP-1c expression is in agreement with previous reports that PKCα can affect the PI3K signaling pathways (Hsu et al., 2018; Sipeki et al., 2006). The detailed molecular mechanism via which PKCα regulates SREBP-1c maturation is still unknown. Taken together, our data suggest that PKCα mainly mediates PA-induced SREBP-1c maturation.
We found that GPRC6A but not GPR120 knockdown led to reduced SREBP-1c expression and maturation. Though previous reports have shown that GPR120 is a sensor of long-chain FAs, till now, there were only a few reports on the role of GPR120 in MECs. A previous report showed that stearic acid suppressed mammary gland development via activation of GPR120 and subsequent inhibition of PI3K (Meng et al., 2017). Another report showed that GPR120 activation induced migration and epithelial–mesenchymal transition of breast cancer cells (M. Zhang & Qiu, 2019). Our data suggest that GPR120 is not involved in milk fat synthesis in BMECs, which is different from these two reports. A recent report shows that GPR120 plays a key role to protect cells from apoptosis (Zhi et al., 2021). The role of GPR120 in BMECs still needs to be determined. In this study, we did not determine the role of GPR40, which is also a critical GPCR for FA sensing (Kimura et al., 2020). The role of GPR40 in BMECs also needs to be explored in future studies.
The ligands of GPRC6A are not fully discovered, though it is considered a multiligand GPCR (Pi et al., 2018, 2016). We found that PA promoted GPRC6A expression and plasma membrane localization, suggesting that GPRC6A might be activated by PA stimulation. It is not known and needs to be explored in the future study whether GPRC6A is a receptor of PA. We further determined that GPRC6A mediated the stimulation of PA on PI3K and PKCα activation and subsequent SREBP-1c expression and activation, showing that GPRC6A controls lipid synthesis via the PI3K/PKCα-SREBP-1c signaling pathways. To our knowledge, this is the first report that a FA functions in lipid synthesis via the GPRC6A signaling. It has not been reported the regulation of GPRC6A on PKCα activation, and our data suggest that the GPRC6A-PKCα signaling might be new signaling to SREBP-1c maturation.
In this study, we explored the molecular mechanism of FA signaling to SREBP-1c expression and maturation for lipid synthesis in BMECs. FAs can also stimulate lipid synthesis in BMECs via activation of transcription factor peroxisome proliferator-activated receptor gamma (PPAR). PPAR isotypes can bind and sense long chain FAs and thereby enhance the pathways for TGs synthesis including SREBP-1c expression (Bionaz et al., 2015; Nakamura et al., 2014). The PPAR isotypes can be activated by long-chain FAs and control the expression of genes involved in lipid metabolism (Bionaz et al., 2013, 2015; Osorio et al., 2016). Recent evidence shows that PA is a key activator of PPAR isotypes in bovine fetal hepatocytes, and PA can activate PPARγ and PPARδ but not PPARα in BMECs (Busato & Bionaz, 2020, 2021). The fact that PA promotes lipid synthesis through both the GPRC6A and PPAR signaling pathways might reflect the molecular mechanism via which cells can sense both extracellular and intracellular PA levels. The connection between these two mechanisms is worth to be explored in future studies.
In summary, the current study uncovers that PA promotes SREBP-1c expression and maturation and lipid synthesis in BMECs via the GPRC6A-PI3K/PKCα signaling pathways. Our findings thus provide a fundamental understanding of GPRC6A in PA-stimulated lipid synthesis and uncover the signaling pathway associated with PA signaling to SREBP-1c expression and maturation.
ACKNOWLEDGMENT
This study was supported by grants from the National Natural Science Foundation of China (31671473).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all datasets generated for this study are included in the article.




