Extracellular vesicles from bone marrow mesenchymal stromal cells of severe aplastic anemia patients attenuate hematopoietic functions of CD34+ hematopoietic stem and progenitor cells
Abstract
Mesenchymal stromal cells (MSC) regulate hematopoiesis in the bone marrow (BM) niche and extracellular vesicles (EVs) released by BM-MSC are important mediators of the cross-talk between BM-MSC and hematopoietic stem and progenitor cells (HSPC). We have previously demonstrated that BM-MSC of severe aplastic anemia (SAA) patients have an altered expression of hematopoiesis regulatory molecules. In the present study, we observed that CD34+ HSPC when cocultured with BM-MSC EVs from aplastic anemia patients exhibited a significant reduction in colony-forming units (p = .001), cell proliferation (p = .002), and increased apoptosis (p > .001) when compared to coculture with BM-MSC EVs from controls. Collectively, our results highlight that EVs derived from the BM-MSC of SAA patients impair the hematopoiesis supporting function of HSPC.
1 INTRODUCTION
Acquired aplastic anemia (AA) is a bone marrow (BM) failure disorder characterized by peripheral pancytopenia and BM hypocellularity due to profound reduction of the hematopoietic stem and progenitor cells (HSPC) (Medinger et al., 2018). Though the pathobiology of the acquired idiopathic AA is still not completely understood, but in most cases there appears to be an immune-mediated destruction of hematopoietic cells and alteration in the BM microenvironment (Medinger et al., 2018). Mesenchymal stromal cells (MSC) are a key cells in the BM microenvironment and are involved in maintaining hematopoietic homeostasis in the BM niche by regulating HSPC proliferation, homing, differentiation and survival (Walenda et al., 2010). In AA, there are scattered reports on the morphological and functional abnormalities of the BM-MSCs (Chaturvedi et al., 2018; Hamzic et al., 2015; Huo et al., 2020; Li et al., 2012). We have previously demonstrated that BM-MSC of AA patients have altered expression of hematopoiesis regulatory molecules, thus highlighting their altered ability to support hematopoiesis (Chaturvedi et al., 2018).
However, the mechanism by which the BM-MSC affects HSPC functions remains unclear. Extracellular vesicles (EVs) are biologically active nano-scaled vesicles which carry different proteins, soluble factors, and microRNAs (miRNAs) cargoes, and are crucial mediators of cell-to-cell communication. Recently, it has been shown that EVs from BM-MSC regulate the hematopoietic functions of HSPC in physiological conditions (Batsali et al., 2020; De Luca et al., 2016; Preciado et al., 2019; Stik et al., 2017). Further, it has been demonstrated that EVs released by the BM-MSC alter the functional properties of HSPC in malignant conditions (Batsali et al., 2020). Thus, it could be hypothesized that EVs released by BM-MSC of AA patients may have an adverse effect on the hematopoietic supporting functions of HSPCs, and thereby a pathological implication in the disease. Therefore, the aim of the present study was to evaluate in-vitro the effect of EVs derived from the BM-MSC of AA patients and controls on hematopoiesis supporting functions of CD34+ HSPCs.
2 MATERIALS AND METHODS
2.1 BM-MSC culture and characterization
BM-MSC were cultured from 5 ml BM aspirates obtained from 10 newly diagnosed severe AA patients (SAA), as per Camitta's criteria (Camitta et al., 1982) and eight healthy patients (controls), after obtaining their informed consent (Supporting Information: Figure 1). The BM-MSC were characterized for the presence of their surface makers and their differentiation potential to adipocytic and osteocytic lineages.
2.2 Isolation and characterization of EVs from BM-MSC of controls and SAA patients
EVs were isolated using Total Exosome Isolation (TEI) Reagent (Invitrogen™) from 3rd passage BM-MSC of SAA patients and controls. In brief, cultured BM-MSC were supplemented with conditioned medium containing 10% Exo-Depleted fetal bovine serum (Gibco), 1% Glutamax (Gibco), 1% Penicillin/streptomycin (Gibco), and alpha-MEM (Gibco) at 37°C and 5% CO2 for 48 h. Post 48-h cell culture supernatant was centrifuged at 2000 g for 30 min to remove cell debris, followed by passing through 0.22 µm filter to eliminate bigger vesicles. TEI reagent was then added to the culture supernatant, followed by overnight incubation at 4°C. The next day, the mixture was centrifuged at 10,000 g for 60 min at 4°C and the pellet obtained was dissolved in the desired medium and stored at −20°C. Further, the characterization of EVs was performed in accordance with Minimal information for studies of EVs 2018 (MISEV2018) guidelines by Scanning electron microscopy (SEM), Nanoparticle tracking analysis, Western blot analysis, and flow cytometry. The detailed methodology of characterization of EVs is provided in the Supporting Information: File (S1).
2.3 Coculture experiments
The EVs derived from BM-MSC of SAA patients and controls were cocultured with Umbilical Cord Blood (UCB)-derived CD34+HSPC in a media supplemented with 100 ng/ml Stem cell factor, 20 ng/ml FMS-like tyrosine kinase 3 ligand (Flt3L) and 20 ng/ml Interleukin-3 (IL-3) in Iscove's Modified Dulbecco's Medium (Gibco) at 37°C at 5% CO2, for 5 days. Post 5 days, HSPC were subjected to functional characterization. For coculture experiment independent samples of 10 SAA and 8 controls were used.
2.3.1 Clonogenic assay
EVs derived from 2 × 105 BM-MSC of SAA patients and controls were cocultured with 2 × 104 CD34+ HSPC in 12 well plate. Post 5 days of Coculture, 1 × 104 HSPC from each group were seeded on to methocult medium. Colony forming Units-Granulocytes Macrophage (CFU-GM) and Burst Forming Units- Erythroid (BFU-E) progenitor cell colonies were counted after 14 days under an inverted microscope.
2.3.2 Cell proliferation
2 × 104 CD34+ HSPC were cocultured with EVs derived from 2 × 105 BM-MSC of SAA patients and controls in a 12 well plate. Cell proliferation was measured based on the cellular DNA content in each group via fluorescent dye binding using CyQUANT NF Cell Proliferation Assay (Life Technologies; Grand Island) following the manufacturer's instruction. Fluorescence intensity corresponding to cell number was measured on Day 0, 2, and 5, respectively, using a fluorescence microplate reader (485 nm excitation λ, 530 nm emission λ).
2.3.3 Apoptosis assay
1 × 105 CD34+ HSPC were cocultured with EVs derived from 1 × 106 BM-MSC of SAA patients and controls, and apoptosis was detected after 5 days using In Situ Cell Death Detection Kit, Fluorescein (Roche) following manufacturer's instruction. TUNEL-positive cells were counted in 10 randomly selected nonoverlapping high-power fields (HPF) (X40 magnification) under confocal microscope (Carl Zeiss LSM 880) and the apoptotic index was expressed as average number of TUNEL-positive cells/HPF.
2.4 Statistical analysis
Values were summarized as mean ± Standard Deviation. The Mann−Whitney U test was used to compare the differences between results. Differences were considered to be statistically significant for values of p < .05. All statistical analysis were done with GraphPad Prism 5.0 software.
3 RESULTS
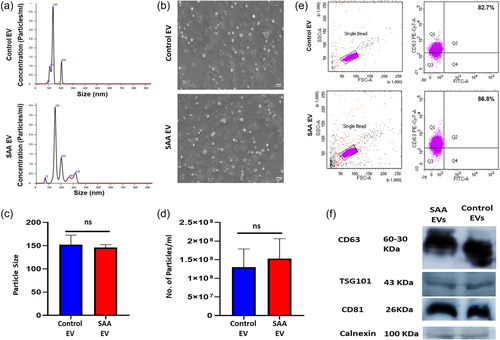
We observed that BM-MSC derived from SAA patients were immunophenotypically similar to BM-MSC of controls (Supporting Information: Figure 2) but had an altered differentiation potential with reduced osteogenic and enhanced adipogenic potential in comparison to controls (Supporting Information: Figure 3). EVs derived from BM-MSCs of SAA patients and controls had a comparable particle size of 146.1 ± 9.5 nm and 152.3 ± 6.2 nm, respectively (p> .05), as analyzed by the Nanoparticle analyzer (Figure 1a). SEM analysis of EVs from control and SAA BM-MSC showed that they had spherical morphology (Figure 1b). Flow cytometric analysis confirmed that EVs derived from the BM-MSC of SAA patients and controls had a comparable expression of CD63, an EV marker (81.90 ± 4.77% vs. 88.13 ± 5.73%) (Figure 1e). Western blot analysis confirmed the expression of other EV specific markers CD63, CD81, TSG101, and negligible expression of calnexin, a negative EV marker in EVs from SAA and control BM-MSC (Figure 1f).
Coculture of UCB derived CD34+ cells with EVs from BM-MSC of SAA patients led to a smaller colony size compared to those cocultured with EVs from control BM-MSC (Figure 2a). Further, CD34+ cells cocultured with EVs from SAA patients had a lower ability to form colonies in comparison to those cocultured with control EVs with CFU of 102.5 ± 8.91 versus 179.1 ± 11.99; p = .001, CFU-GM of 13.10 ± 1.41 versus 21.13 ± 1.96; p = .006 and erythroid colonies (BFU-E) of 90.40 ± 8.82 versus 158.0 ± 10.45; p = .001 (Figure 2b). A comparison of the total number of colonies revealed that CD34+ cells cocultured with SAA EVs had approximately a 2.0-fold decrease in their colony-forming ability in comparison to those cocultured with control EVs (Figure 2b). Moreover, we saw that EVs from BM-MSC of healthy control had an enhanced colony forming ability of CD34+ HSPC compared to EV nontreated CD34+ cells (p = .04) (Figure 2a,b), suggesting that in normal physiological conditions, EVs support the colony-forming ability of CD34+ HSPC.
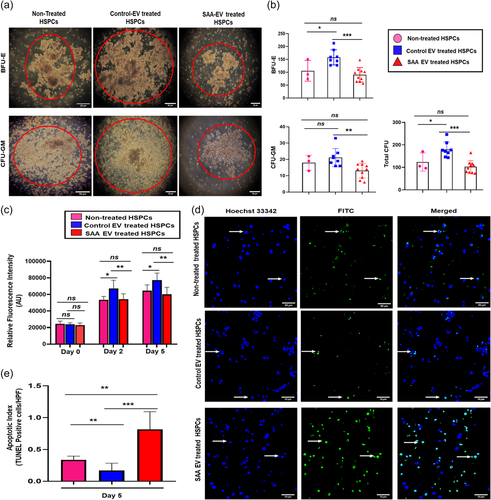
Next, we sought to determine their effect on CD34+ cell survival in-vitro. We observed a significant decrease in the proliferative potential of CD34+ cells treated with EVs from SAA BM-MSC in comparison to those treated with control EVs on Day 2 (p = .008) and consecutively on day 5 (p = .002) (Figure 2c). Furthermore, EVs derived from the BM-MSC of SAA patients lead to increased apoptosis of CD34+ cells with a significantly higher number of TUNEL positive CD34+ cells as compared to those cocultured with control EVs (Figure 2d) resulting in an enhanced apoptotic index (p < .001) (Figure 2e). Additionally, we also saw that in comparison to HSPC treated with healthy control BM-MSC derived EVs, EV nontreated CD34+ HSPC had reduced cell proliferation both on Day 2 (p = .02) and Day 5 (p = .04), with increased apoptosis (p = .009) suggesting that EV from healthy control BM-MSC promotes cell proliferation and reduces apoptosis, thus having a role in cell survival of HSPC.
4 DISCUSSION
We have investigated the effect of EVs derived from the BM-MSC of SAA patients and controls on hematopoietic supporting functions of UCB derived CD34+ HSPC in-vitro. Our study demonstrates that EVs released by the BM-MSC of SAA patients reduce the viability and clonogenicity of HSPC and increases their apoptosis, suggesting their potential pathogenic involvement in hematopoietic dysfunction seen in AA.
BM-MSC are an essential component of the BM microenvironment and are pivotal for the balanced regulation of HSPC functions (Jing et al., 2010; Méndez-Ferrer et al., 2010; Saleh et al., 2015; Walenda et al., 2010). We and a few other studies have reported morphologic, genetic and functional impairment of BM-MSC in acquired AA (Chaturvedi et al., 2018; Hamzic et al., 2015; Huo et al., 2020; Li et al., 2012; Shipounova et al., 2009; Tripathy et al., 2014). Another report shows that BM-MSC of AA patients when cocultured with CD34+ cells, reduced the proliferative and clonogenic potential of CD34+ cells (Hamzic et al., 2015). However, the mechanism by which BM-MSC impairs HSPC functions in AA is still not known. Studies exploring the mechanism of hematopoiesis supporting ability of BM-MSCs have shown that they exert their beneficial effect on HSPCs in a paracrine manner via the release of cytokines, growth factors and EVs (Aqmasheh et al., 2017; Batsali et al., 2020).
EVs mediate cell-cell communication by carrying molecular messages from the parent cells and transferring it to the target cells, eventually modifying their functions. EVs derived from stem cells have been shown to modulate HSPC functions by inducing transcriptional reprogramming via efficient transfer of mRNA and miRNAs (De Luca et al., 2016; Ratajczak et al., 2006). In normal physiological state, MSC-EVs enhance the clonogenicity and proliferation of HSPC and inhibit their apoptosis by regulating the expression of their corresponding genes (De Luca et al., 2016; Preciado et al., 2019). Studies have also shown that BM-MSC derived EVs can potentially ameliorate the HSPC functions in radiation induced BM failure and immune mediated AA mouse models by enhancing the colony formation and proliferation of HSPC as well as reducing apoptosis (Gholampour et al., 2021; Wen et al., 2016). In hematological disorders such as acute myeloid leukemia and myelodysplastic syndrome (MDS), EVs have been reported to disrupt the balance between the proliferation and apoptosis of HSCs (Barrera-Ramirez et al., 2017; Cominal et al., 2019; Horiguchi et al., 2016; Kumar et al., 2018; Muntión et al., 2016). In AA a single study on plasma derived exosomes has shown that the exosomes from AA patients have differential expression of miRNAs which could be potentially involved in HSPC differentiation, proliferation, quiescence and apoptosis (Giudice et al., 2018) We observed in AA patients that the EVs from the BM-MSC have reduced the proliferation and led to an increase in apoptosis of HSPC.
Muntión et al. (2016) have shown that the EVs derived from BM-MSC of MDS patients get incorporated into the HSC and affect their clonogenicity. Similarly, our results also showed that HSPC cocultured with EVs derived from the BM-MSC of SAA patients formed fewer colonies in comparison to those treated with control EVs, thereby suggesting that EVs released by the SAA BM-MSC could potentially alter the clonogenic potential of HSPC. Wen et al. (2016) has shown that BM-MSC could potentially restore the clonogenic potential of HSPC in radiation induced BM failure. A recent study showed in a mouse model of AA that the infusion of healthy BM-MSC EVs reversed the hypoplastic BM condition by restoring the number of HSPC (Gholampour et al., 2021), corroborating with our observation of altered hematopoietic supporting functions of BM-MSC EVs in AA.
In conclusion, we have demonstrated that BM-MSC derived EVs from SAA patients reduce the clonogenicity, proliferation and increase the apoptosis of HSPC highlighting that BM-MSC mediates their functional effects on HSPC through secreted EVs and may be contributing towards the hematopoietic dysregulation in AA. To the best of our knowledge, this is the first study to demonstrate that EVs from BM-MSC of SAA patients significantly reduce the hematopoiesis supporting functions of CD34+ cells. Since EVs are enriched in small protein and miRNAs, our future goal is to identify the miRNAs and proteins cargos, differentially expressed in the BM-MSC EVs of SAA patients and decipher their involvement in AA pathophysiology
AUTHOR CONTRIBUTION
Jyotika Srivastava: Designed and performed the experiments; analyzed the data and wrote the manuscript. Chandra Prakash Chaturvedi: Conceived and designed the experiments; analyzed the data and provided reagents and wrote the manuscript. Shobhita Katiyar: Performed the experiments. Soniya Nityanand: Conceived and designed the study; analyzed the data; provided the reagents; wrote the manuscript; and finally approved it.
ACKNOWLEDGMENT
We would like to extend our thanks to Dr. Khaliqur Rahman, Addl Prof, Dept of Hematology, for helping us to conduct the flow cytometry experiments. We also thank Dr. Ruchi Gupta, Addl Prof, Dept of Hematology, for helping us in categorizing the severity of patients. We would like to express our deep gratitude to all the patients and controls for donating their bone marrow samples. This study was supported by an Extramural Grant of the Department of Biotechnology (DBT) (BT/PR31421/MED/31/407/2019) and SGPGI Intramural Grant (A-14-PGI/76/2018) sanctioned to Prof. Soniya Nityanand. Wellcome Trust DBT India Alliance Grant (IA/I/16/1/502374) sanctioned to Dr. C. P. Chaturvedi. Jyotika Srivastava is a recipient of INSPIRE PhD. Fellowship (IF170881) from the Department of Science and Technology (DST).
ETHICAL STATEMENT
This study is a prospective study which has been approved by the Institutional Ethics Committee (IEC-2018-173-EMP-107) and Institutional Committee for Stem Cell Research (ICSCR–2019-02-EMP-08) of the Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
All the data related to the study has been included in the article. Any further information related to the study can be obtained from the corresponding author upon reasonable request.




