Chrysin sensitizes osteosarcoma cells against TRAIL-induced apoptosis
Abstract
Identifying novel curative and preventive approaches that can specifically target the osteosarcoma cells (OS) without affecting the normal cells is appreciable. The aim of this study is to investigate the combined effect of chrysin as an apigenin analog with high therapeutic potential and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on the treatment of Saos-2 and MG-63 cells. Cell viability were determined using MTT method. The rate of apoptosis was assessed by enzyme-linked immunosorbent assay (ELISA) cell death assay and caspase 8 activity assays. The messenger RNA (mRNA) and protein evaluation of candidate genes include Bcl-2, XIAP, c-IAP1, c-IAP2, and c-FLIP were accomplished before and after the treatment by quantitative real-time polymerase chain reaction (PCR) and Western blot analysis, respectively. Our results showed that chrysin synergistically increased the cytotoxic effects of TRAIL as follows: Chrysin plus TRAIL > TRAIL > Chrysin. Chrysin could sensitize both cells against the TRAIL-induced apoptosis, amplify the caspase 8 activity and this outcome is achieved by decreasing the expression levels of antiapoptotic genes. Our findings suggest that Chrysin can sensitize the OS cell lines against TRAIL through induction of the death receptor pathway. Moreover, the combinational therapy of these agents might be the promising therapeutic regimen for improving the clinical efficacy of TRAIL-induced apoptosis in patients with OS.
1 INTRODUCTION
Osteosarcoma is ranked among the most malignant and aggressive bone cancer manifested in children and adolescents (Mirabello et al., 2009). Therapeutic strategies for osteosarcoma include neoadjuvant chemotherapy and surgery (Jaffe, 2009). Despite promising improvements in cancer therapy in the past few decades, survival rates of patients suffering from osteosarcoma are considerably low, such that patients with metastases have 5-year survival less than 30% (Gorlick et al., 2013). The development of resistance against chemotherapeutic agents and metastasis to vital organs are two most important reasons for the failure of current therapeutic modalities (A. J. Chou & Gorlick, 2006). Therefore, recently, researchers have focused on finding novel and effective approaches to combating drug resistance in osteosarcoma, as well as reversing adverse clinical outcomes in these patients.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), belonging to the tumor necrosis factor (TNF) cytokine superfamily is a ligand for the death receptor (DR)-4 and DR-5 on the cell surface, which are responsible for the initiation and stimulation of the extrinsic apoptotic pathway. TRAIL represents specific and potent cytotoxicity in tumor cells. Therefore, monoclonal antibodies against DR-4 and DR-5, as well as recombinant TRAIL are among the most effective and novel therapeutic agents used for treating a broad range of cancer types (Holoch & Griffith, 2009). However, osteosarcoma cells have been reported to develop resistance against TRAIL, which means for gaining hopeful results in inducing apoptosis, other chemotherapeutics should be used in combination with TRAIL (Hotta et al., 2003; Mirandola et al., 2006). Therefore, recent studies have demonstrated the efficacy of using natural compounds such as chrysin in overcoming drug resistance and increasing the efficacy of conventional chemotherapeutics such as TRAIL in numerous cancers. As a natural flavonoid, chrysin is found in different plant extracts, propolis, and honey. Various pharmacological and biological features such as anti-inflammatory, antioxidant, antiproliferative, proapoptotic, and anticancer have been introduced for chrysin (Pushpavalli et al., 2010; Shin et al., 2009). An accumulating number of previous studies have reported that chrysin exerted potent anticancer effects in numerous cancer types through various mechanisms including apoptosis induction, inhibition of cellular proliferation, and modulating different signaling pathways (Li et al., 2010; Maasomi et al., 2017; Miyamoto et al., 2006; Ryu et al., 2017; Yang et al., 2013). In addition, recent studies have demonstrated that a combination of chrysin effectively reverses the chemoresistance to chemotherapeutic agents such as cisplatin, 5-flourouracil, oxaliplatin, and deocetaxel (Ghamkhari et al., 2019; Lee et al., 2021; Li et al., 2015; Lin et al., 2018). To the best of our knowledge, the efficacy of chrysin in increasing the chemosensitivity of osteosarcoma cells to TRAIL has not been investigated. Therefore, we aimed to investigate the combined effect of chrysin and TRAIL on the proliferation and apoptosis of Saos-2 and MG-63 cells.
2 MATERIAL AND METHODS
2.1 Cell lines and cell culture
Saos-2 and MG-63 human osteosarcoma cells (China Institute Cell Bank) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 100 unit/ml penicillin/streptomycin and incubated at 37°C with 5% CO2. Antibodies were obtained from Abcam. For both cell lines, small quantities of the same frozen cells were thawed and applied in all experiments in the same number of passages (5–10 passages) to ensure lack of variations in cell population.
2.1.1 Cell viability assay
Cell viability following treating cells with TRAIL (Sigma Aldrich) and chrysin Cayman Chemical in alone or in combination, MTT assay was performed. For this purpose, after culturing Saos-2 and MG-63 cells in 96-well plates, cells were treated with 0–100 μM of chrysin, in alone or in combination with 100, 500, 1000, 2000, and 4000 nM TRAIL. After 48 h incubation, 50 µl of 5 mg/ml MTT solution was added and cell were incubated at 37°C with 5% CO2 for 4 h. After removing MTT solution, DMSO was added and the formed violet crystals were dissolved. The OD was measured at 570 nm using a microplate reader.
2.2 Analyzing the combination effect of TRAIL and chrysin
Measuring the coefficient of drug interaction (CDI) value was applied to determine the interaction between TRAIL and chrysin in Saos-2 and MG-63 cells (T. -C. Chou & Talalay, 1984). For this purpose, the following equation was used: CDI = AB/(A×B).
Where A and B are the survival rate of cells treated with TRAIL and chrysin in relevance to their control groups, and AB is the survival rate cells treated with the combination of TRAIL and chrysin in relevance to their control groups. CDI < 1, CDI = 1, and CDI > 1 show synergistic, additive and antagonistic interactions between two drugs.
2.3 ELISA cell death assay
The effects of chrysin on sensitizing Saos-2 and MG-63 cells to the TRAIL-induced apoptosis were measured using Cell Death Detection ELISA kit (Roche Applied Science). A density of 1 × 104 cells per well were seeded in 96-well plates. Then, the cells were treated with chrysin, TRAIL, and their combination for 48 h. Cell death were evaluated using commercial ELISA kit in accordance to the manufacturer's protocol.
2.4 Caspase-8 activity assays
Caspase-8 colorimetric assay was used to measure the effects of chrysin and TRAIL combination on the activity of Caspase-8 activity, as a key enzyme in triggering apoptosis. After treating cells with chrysin and TRAIL and their combination, Caspase-8 colorimetric assay kit was used in accordance to the manufacturer's instruction (BioVision). This assay is based on the production of chromophore p-nitroanilide (pNA), which is cleaved from the labeled substrate involved in recognizing the individual activation sites of caspase-8. pNA was detected in samples spectrophotometrically at 405 nm.
2.5 Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA of all groups was extracted from Saos-2 and MG-63 cells with Accuzol specific RNA extraction kit (Bioneer). After evaluating the quality and quantity of RNA samples, RNAs were subjected to reverse transcription to complementary DNA (cDNA) using Bioneer kit (Korea) in accordance to manufacturer's protocols. PCR was applied by using the SYBR Green and specific primers on Rotor-Gene™ 6000 system (Corbett Life Science). Relative gene expression of target genes was calculated using method. β-actin was used as an internal control. The primer sequence for β-actin, Bax, Bcl-2, Caspase-9, XIAP, c-IAP1, c-IAP2, and c-FLIP were presented in Table 1.
| Gene | Primer sequence |
|---|---|
| BCL-2 | F 5′-GAGCGTCAACCGGGAGATGTC-3′ |
| R 5′-TGCCGGTTCAGGTACTCAGTC-3′ | |
| BAX | F 5′-GACTCCCCCCGAGAGGTCTT-3′ |
| R 5′-ACAGGGCCTTGAGCACCAGTT-3′ | |
| cIAP1 | F 5′- TGTTGTCAACTTCAGATACCACTGG -3′ |
| R 5′- CATCATGACAGCATCTTCTGAAGA-3 | |
| cIAP2 | F 5′- GGACAGGAGTTCATCCGTCAAG -3′ |
| R 5′- TGGATAATTGATGACTCTGCATTTTC -3 | |
| XIAP | F 5′- GACAGTATGCAAGATGAGTCAAGTCA-3′ |
| R 5′- GCAAAGCTTCTCCTCTTGCAG -3′ | |
| c-FLIP | F 5′- CCAGAGTGTGTATGGTGTGGAT -3′ |
| R 5′- TCTCCCATGAACATCCTCCTGAT -3′ | |
| Caspase-9 | F 5′- TTGTTGTTTGGGTAATGTATTGA-3′ |
| R 5′- CAACCCTACTTAACTTAACCCTACTCA-3′ | |
| β-actin | F 5′- TCCCTGGAGAAGAGCTACG-3′ |
| R 5′- GTAGTTTCGTGGATGCCACA-3′ |
- Abbreviation: PCR, polymerase chain reaction.
2.6 Western blot analysis
For evaluating the protein expression levels of target genes, western blot analysis was applied. For this purpose, cells were lysed using a cold RIPA buffer. Bradford assay and bovine serum albumin (BSA) as standard were used to quantify the protein concentrations in cell lysates. A total of 50 μg of cell lysate was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked by incubation in skimmed milk. After overnight incubation with primary antibodies against target proteins including Bcl-2, XIAP, c-IAP1, c-IAP2, and c-FLIP, membranes incubated again with horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h. The bands were developed by the enhanced chemiluminescence (ECL) reagents and quantified with Image J software.
2.7 Statistical analysis
SPSS software was used to perform statistical analysis. Results were presented as mean ± SD from at least three independent experiments. The significance of differences between groups was estimated by the ANOVA. p-value of less than .05 was considered to be statistically significant.
3 RESULTS
3.1 Cell viability in cell treated with chrysin and TRAIL alone or combination
Results from MTT assay showed that chrysin exerted potent antiproliferative effects in both cell lines (Figure 1). In Saos-2 cells, the IC50 value for chrysin was 45.1 and 38.5 μM in Saos-2 and MG-63 cell lines, respectively. In addition, TRAIL had also a cytotoxic impact on both cell lines. The IC50 value of TRAIL was 1782 nM in Saos-2 cells and 1750 nM in MG-63 cell lines (Figure 1a,c). Not surprisingly, the combination of 45 μM chrysin with different concentrations of TRAIL resulted in more potent inhibition in the Saos-2 cell lines, such that the IC50 value of TRAIL decreased to 1358 nM (Figure 1b). Similar results were found in MG-63 cell lines. We showed that 38 μM combined with TRAIL led to potent inhibitory effects on this cell line. The IC50 value for TRAIL was reduced to 1276 nM, when cells were treated with a combination of chrysin and TRAIL (Figure 1d). In both cell lines, the combined effects of TRIAL and chrysin were also synergistic with the CDI values of less than 1 in all concentrations of TRAIL (Figure 1b,d). These results showed that chrysin decreased the IC50 value of TRAIL and increased the cytotoxic effects of TRIAL on osteosarcoma.
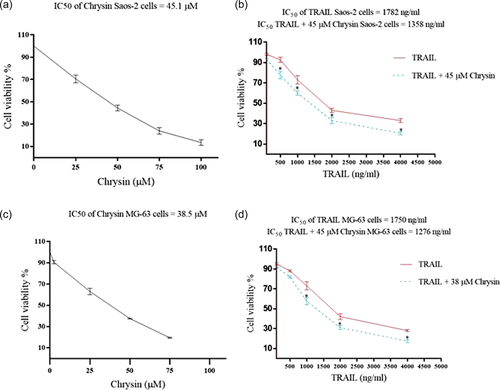
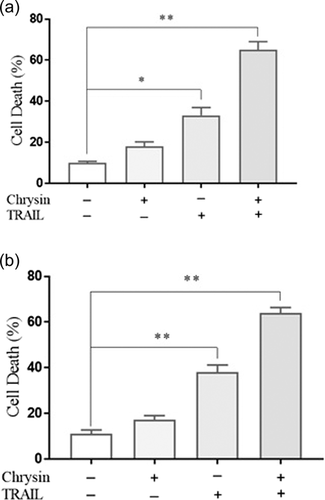
3.2 Chrysin and effects on TRAIL-induced apoptosis in Saos-2 and MG-63 cells
For approving results from the MTT assay, the apoptosis was also evaluated in osteosarcoma cell lines via cell death ELISA assay. As shown in Figure 2, results from ELISA assay, chrysin, and TRAIL treatments resulted in a significant increase in the cell death percentage in both cell lines in comparison to nontreated cells (p < .05). The combination of chrysin and TRAIL in Saos-2 and MG-63 significantly elevated the number of apoptotic cells, when compared with cells treated with chrysin or TRAIL, in alone. This finding approved that combined treatment of osteosarcoma cells with chrysin and TRAIL has potent proapoptotic effects, which suggested that chrysin sensitized osteosarcoma cells to TRAIL-induced apoptosis.
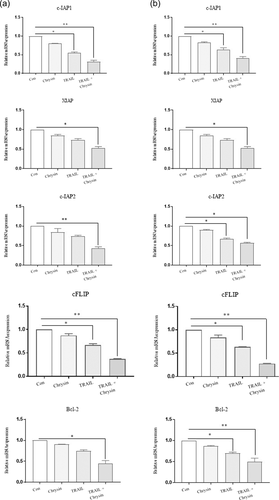
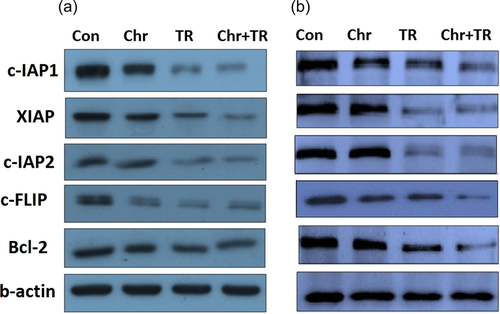
3.3 The effects of chrysin on the TRAIL-induced apoptosis-related genes and protein expression levels
In the next step of the present study, the mRNA and protein expression levels of Bcl-2, XIAP, c-IAP1, c-IAP2, and c-FLIP, as major mediators of apoptosis were assessed in Saos-2 and MG-63 cells treated with chrysin, TRAIL and their combination (Figures 3 and 4). We found that in both cells the mRNA and protein expression levels of Bcl-2, XIAP, c-IAP1, c-IAP2, and c-FLIP were downregulated upon treatment with chrysin or TRIAL in comparison to nontreated cells. Moreover, the combination of both interventions resulted in a more potent inhibitory effect on the mRNA and protein expression levels of all antiapoptotic mediators (p < .05). Therefore, chrysin induced apoptosis by inhibiting the antiapoptotic mediators in osteosarcoma cells.
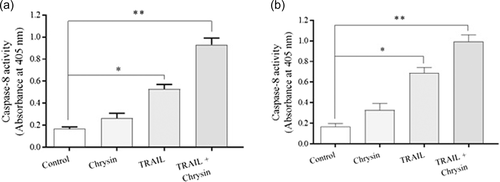
3.4 Chrysin promotes TRAIL-induced apoptosis through caspase 8-dependent in osteosarcoma cells
To evaluate the underlying mechanisms for sensitizing effects of chrysin on TRAIL-induced apoptosis in osteosarcoma cells, the caspase-8 activity was also evaluated. As represented in Figure 5, treatment with TRAIL significantly enhanced the activity of Caspase-8 in both osteosarcoma cell lines (p < .05). In cells treated with the combination of chrysin and TRAIL, a robust elevation was observed in the Caspase-8 activity (p < .05), which means that chrysin promoted TRAIL-induced apoptosis through Caspase-8-dependent manner in Saos-2 and MG-63 cells of osteosarcoma.
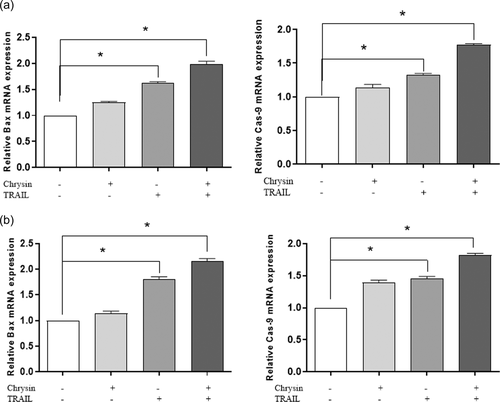
3.5 Chrysin promotes TRAIL-induced apoptosis through activating apoptosis intrinsic pathway in osteosarcoma cells
In addition to caspase-8, which is one of the main components of the apoptosis extrinsic pathway, Bax and Caspase-9 expression levels were also evaluated via qRT-PCR, to assess the involvement of chrysin on the apoptosis intrinsic pathway. As shown in Figure 6, TRAIL treatment led to a significant increase in the mRNA expression levels of Bax and Caspase-9 in Saos-2 and MG-63 cells (p < .05). On the other hand, combination treatment of both cell lines with chrysin and TRAIL elevated the Bax and Caspase-9 expression levels more potently, in comparison to mono-treatments (p < .05: Figure 6). This finding implies that chrysin may enhance TRAIL-induced apoptosis in osteosarcoma cells via both apoptosis intrinsic and extrinsic pathways and targeting key components of both pathways including Bax, Bcl-2, Caspase-8, and Caspase-9.
4 DISCUSSION
It is now well-known that the appearance of drug resistance against conventional chemotherapeutic agents frequently occurs in cancer cells, which creates a serious burden against the successful and efficient killing of cancer cells, hence tumor recurrence in the next years. Therefore, recent investigations have been focused on combination therapy, in which conventional chemotherapeutics are applied in combination with safe, more effective, and in more cases natural compounds, to increase the cytotoxic effects of drugs, overcome drug resistance, and decrease sever drug-related side effects (Kasala et al., 2015; Yardley, 2013). In this study, we showed that the combination of chrysin with TRAIL in osteosarcoma cells led to significant enhancement in the cytotoxic effects of TRAIL via sensitizing cancer cells to TRAIL-induced apoptosis, downregulating major antiapoptotic mediators in a Caspase-3 dependent manner.
The anticancer effects of chrysin are extensively studied in various cancer types, such as osteosarcoma. For example, Naso et al. reported that treatment of UMR106 rat osteosarcoma cell line with 25 μM chrysin significantly inhibited cancer cells proliferation in a dose-dependent manner, which is mediated to increasing oxidative stress in cancer cells (Naso et al., 2010). In another study, Leon et al. showed that embedding chrysin in a vanadyl(iv) complex led to increasing in oxidative stress-mediated apoptosis and hence a potent proapoptotic effect in osteosarcoma MG-63 cell lines (Leon et al., 2013). Our results are in agreement with these studies, which showed the antiproliferative impact of chrysin on Saos-2 and MG-63 cell lines.
Moreover, the sensitizing effects of chrysin to numerous chemotherapeutic agents were also investigated on various cancer cells. For instance, Li et al. demonstrated that chrysin in combination with cisplatin exerted a potent anticancer effect on Hep G2 cells (Li et al., 2015). It sensitized cancer cells to cisplatin via stabilizing p53 protein and ERK1/2- mediated phosphorylation of p53. In numerous models of acquired and primary drug resistance, chrysin is also indicated to induce cell death. Wang et al. showed reversing drug resistance against Adriamycin and cisplatin in non-small cell lung cancer cells via suppressing interleukin-6 induced expression of dihydrodiol dehydrogenases (Wang et al., 2007). In doxorubicin-resistant BEL-7402 cells, chrysin downregulated Nrf2, AKR1B10 multidrug resistance-associated protein-5, and heme oxygenase-1, as well as suppressed the ERK and PI3K-Akt signal transductions, which ultimately sensitized these resistance cancer cells to doxorubicin (Gao et al., 2013).
Another potential mechanism for chrysin in combating drug resistance is inhibition of drug efflux pumps, hence promoting the accumulation of cytotoxic drugs in cancer cells and restoring sensitivity to them (Zhang et al., 2004). In addition, chrysin is also demonstrated to weaken cells antioxidant defense system by depleting cellular glutathione levels in PC-3 and HL-60 cells (Kachadourian et al., 2007). Mehdi et al. reported the efficacy of chrysin in overcoming resistance to TRAIL in lung cancer cell lines. They showed that chrysin increased TRAIL-induced apoptosis upregulating major apoptosis mediators such as including Caspase-3, 8, 9 and Bax and downregulating Bcl-2. In agreement with these studies, we found that chrysin sensitized osteosarcoma cells lines to TRAIL via inducing apoptosis and downregulating antiapoptotic mediators in Caspase-8 dependent manner.
5 CONCLUSION
Our finding demonstrated the apoptosis augment in cells treated with the combination of chrysin and TRAIL in osteosarcoma Saos-2 and MG-63 cells via suppressing the expression of antiapoptotic genes and proteins in the caspases-dependent pathway. Therefore, chrysin may be an appropriate candidate for combating drug resistance against TRAIL and increasing the cytotoxic effects of this agent, as well as reducing TRAIL-related side effects. However, further detailed investigations are required to elucidate the underlying mechanisms.
ACKNOWLEDGMENT
Thanks for supporting from Shaanxi provincial key research and development program (2021SF-353).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was not in human or animal samples.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




