DNA methylation influences the CTCF-modulated transcription of RASSF1A in lung cancer cells
Abstract
Ras-association domain family 1A (RASSF1A) is one of the most methylated genes in lung cancer (LC). We investigate whether the high DNA methylation level of RASSF1A can relieve the resistance of RASSF1A to LC by inhibiting RASSF1A's transcription factor binding to RASSF1A. RASSF1A expression in tissues and cells was tested utilizing quantitative real-time polymerase chain reaction (qRT-PCR), and Western blot. RASSF1A expression and RASSF1A methylation level in LC cells exposed to 5-Aza-dc were assessed by qRT-PCR and quantitative methylation-specific PCR. The association between CTCF and RASSF1A was assessed using hTFtarget, ChIP, and luciferase reporter gene analysis. The effects of 5-Aza-dc, CTCF, and RASSF1A on cell biological behaviors and epithelial-mesenchymal transition (EMT)-related markers were assessed by cell function experiments and Western blot. Moreover, we constructed the xenograft tumor and pulmonary nodule metastasis models, and assessed tumor volume and weight. RASSF1A expression and pulmonary nodule metastasis were tested utilizing qRT-PCR, Western blot, and H&E staining. RASSF1A was under-expressed in LC tissues and cells. 5-Aza-dc enhanced RASSF1A level and weakened RASSF1A methylation level in LC cells. RASSF1A silencing neutralized 5-Aza-dc-mediated repressing effects on LC cell biological function and EMT. The loss of CTCF binding to RASSF1A in LC cells was associated with DNA methylation. The effect of 5-Aza-dc on RASSF1A level, LC cell malignant behaviors, and EMT-related factors were strengthened by CTCF upregulation. RASSF1A overexpression suppressed LC tumor growth and pulmonary nodule metastasis in vivo. DNA methylation blocked the modulation of RASSF1A expression by CTCF and relieved the resistance of RASSF1A to LC.
1 INTRODUCTION
Lung cancer (LC) is a malignancy originating from bronchial mucosa or glands (Bao et al., 2019). LC is a malignancy with high morbidity and mortality in the world, which seriously threatens human life and health (Siegel et al., 2020). The report pointed out that the vast majority of LC patients are in the middle and advanced stage of cancer at the time of diagnosis, especially for LC patients who have distant metastasis and cannot be removed at the time of diagnosis, their treatment is more difficult, resulting in low survival rate (Lee et al., 2018). The 5-year survival rate of LC varies by country and region, ranging from 4% to 17% (Guo et al., 2019; Hirsch et al., 2017). Therefore, early effective and accurate screening and diagnosis of LC is an urgent problem in the clinic. At present, the early screening methods for LC are mainly chested X-ray examination, cytology examination, imaging examination, bronchoscopy, and lung biopsy, but the sensitivity is not ideal, which is very difficult for the early diagnosis of LC (Xu et al., 2021). Therefore, the detection of molecular markers with high sensitivity and good specificity of specimens obtained by noninvasive or minimally invasive methods is particularly important in the auxiliary diagnosis of early LC.
In recent years, through the continuous in-depth study of LC, the epigenetic regulation mechanism, especially the DNA methylation mechanism, has attracted the attention of many researchers at home and abroad. More and more evidence documented that DNA methylation is crucial for the regulation of gene expression and the maintenance of cell characteristics, which is tightly correlated with the genesis and development of human malignant tumors, including LC (Chakravarthi et al., 2016; Khan et al., 2015). With the development of DNA methylation detection technology, the research on DNA methylation in LC now focuses more on DNA methylation in liquid biopsy (Millares et al., 2014; Zhang et al., 2017). For example, abnormal changes in the methylation status of related gene promoter CpG islands can be detected in body fluids such as plasma, sputum, and bronchoalveolar lavage fluid of LC patients, making it a molecular marker for noninvasive or minimally invasive early diagnosis of LC (Hubers et al., 2014; Kneip et al., 2011; Leng et al., 2012). Relevant evidence unveiled that ras-association domain family 1A (RASSF1A) is tightly related to the initiation and development of various malignant tumors, and RASSF1A is one of the most methylated and common genes in tumors (Wei et al., 2015). RASSF1A, as a negative regulator gene in the Ras activation pathway, manifests a major function in modulating cell proliferation and apoptosis (Agathanggelou et al., 2005; Chen et al., 2017). In previous studies, we have demonstrated that RASSF1A has a high DNA methylation level in LC tissues and is related to a poor prognosis of LC, suggesting that DNA methylation of RASSF1A has a good value in the differential diagnosis of LC. Current studies illuminated that transcription factors are sensitive to DNA methylation, and DNA methylation can inhibit the binding of transcription factors to genes, thereby affecting gene expression (Héberlé & Bardet, 2019). However, it remains unclear whether the high DNA methylation level of RASSF1A can relieve the resistance of RASSF1A to LC cells by weakening RASSF1A's transcription factor binding to RASSF1A.
DNA methyltransferase inhibitor (5-Aza-dc) has a similar structure to pyrimidine, can covalently bind DNA methyltransferase to inhibit its enzymatic activity and restore the anticancer function of methylated CpG islands (Park et al., 2018). 5-Aza-dc has been ratified by the US Food and Drug Administration as a demethylation agent for the treatment of malignant tumors (Issa et al., 2005). This study intends to observe the methylation status of the RASSF1A promoter in LC, further apply 5-Aza-dC to reverse the methylation expression of RASSF1A, analyze its effect and mechanism on LC, aiming to provide experimental evidence for early clinical diagnosis and treatment of LC.
2 MATERIALS AND METHODS
2.1 Patients and LC tissues
A total of 72 LC samples and adjacent samples were harvested from participants at YUE BEI PEOPLE'S HOSPITAL. We quickly frozen the collected tissues in liquid nitrogen and utilized them for quantitative real-time polymerase chain reaction (qRT-PCR).
2.2 Cell culture and treatment
LC cell lines H1395 (C1048), H1703 (C1059), A549 (C1002), and H1299 (C1122) and human embryonic lung cell line MRC5 (C1131) were available from WHELAB. RPMI-1640 complete medium (M0201A, WHELAB) was utilized for the growth of the above cells. Cells were kept at a cell incubator (Herocell 180; RADOBIO).
LC cells were first stimulated with 1, 5, and 10 µM 5-Aza-dc (189825; Sigma-Aldrich) for 48 h to observe the expression of RASSF1A and the methylation level of RASSF1A in each group. Next, to check the impact of RASSF1A on LC cells exposed to 5-Aza-dc, cells transfected with or without RASSF1A short hairpin RNA (shRASSF1A) or its negative control (shNC) were exposed to 10 µM 5-Aza-dc. Then, to analyze the role of CTCF on LC cells mediated by 5-Aza-dc, cells were transfected with or without CTCF overexpression or NC in the presence or absence of 10 µM 5-Aza-dc.
2.3 Transfection
YouBio offered pSilencer 3.0-H1 vector (VT1392). The pSilencer 3.0-H1 vector was applied to construct shRASSF1A, with an empty vector as the corresponding control RNA (shNC). The plasmids for CTCF overexpression and RASSF1A overexpression were synthesized via inserting full-length CTCF and RASSF1A into pcDNA3.1(+) vector (VT1001; YouBio). Empty pcDNA3.1 vector was exploited to NC. LC cells (2 × 105 per well) were appended to 24-pore plates all night. Transfection was done utilizing Lipo6000 (C0526; Beyotime).
2.4 Cell counting kit-8 (CCK-8)
LC cells (8000 per well) were grown in 96-pore plates for 1 day. Next, cells were stimulated as mentioned previously. Then, cells were additionally reacted for 4 h after cells were exposed to 10 μl CCK-8 solution (C266180; Aladdin). In the end, the optical density value at 450nm was recorded with the help of an enzyme immunoassay analyzer (LX800; BioTek) at 24, 48, and 72 h.
2.5 Scratch assay
When LC cells (10,000 per well) grew to 80% fusion in 6-pore plates, the monolayer was injured by scratching the cells with a pipette tip. After removing broken cells, cells photographs were acquired with an inverted microscope 100 times (IX71; Olympus) at 0 and 48 h. The distances were estimated utilizing Image J (version 1.48; National Institutes of Health).
2.6 Transwell assay
LC cell suspension without fetal bovine serum (FBS) were loaded into the upper chamber of Transwell (3422; Corning) which was coated with Matrigel (356234; BD Biosciences) in advance, while the medium blended with 10% FBS was appended to the lower chamber. After being reacted for 24 h, LC cells invaded into the down chamber was fixed with 4% paraformaldehyde (E672002; Sangon) before staining with 0.1% crystal violet (E607309; Sangon). Thereafter, the penetrated cells were observed, imaged, and calculated with the microscope 250 times.
2.7 QRT-PCR
Total RNA from cultivated cells or tissues was isolated with the help of the RNA extraction kit (LS1040; Promega). After examining RNA concentration and purity, the one-step RT-qPCR system (A6020; Promega) was exploited for the analysis of qRT-PCR. The amplification reaction was carried out on an Mx3005P RT-PCR System (Stratagene). GAPDH was acted as the normalizer. The quantification of RASSF1A and CTCF were conducted with the method. Table 1 exhibited the primer sequences.
| Name | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| RASSF1A | TCCTGCAAGGAGGGTGGCTTC | GGCTGGGAACCCGCGGTG |
| CTCF | TCAGTGCAGTTTGTGCAGTTA | TTCCCCTGAATGGGTTCTCAT |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
2.8 Western blot
RIPA Lysis Buffer (G2002; Servicebio) was added to cells or tissues. After being centrifuged, the acquired supernatant was examined with a BCA kit (G2026; Servicebio). Following isolation of the quantified protein by 10% SDS-PAGE, they were electroblotted onto nitrocellulose membranes and then exposed to 5% skimmed milk. After being incubated with primary antibodies at 4°C overnight, the secondary antibody (anti-rabbit; 1:5000; ab6721; Abcam) was taken to further treat the membranes. Following reaction at 37°C for 60 min, the membranes with ECL solution (G2014; Servicebio) were detected with an image analysis system (5200; Tanon). The primary antibodies of RASSF1A (1:8000; ab126764; 40kDa), RUNX2 (1:1000; ab23981; 60 kDa), MMP-3 (1:20000; ab52915; 50 kDa), E-cadherin (1:50000; ab40772; 97 kDa), N-cadherin (1:20000; ab76011; 100 kDa), and GAPDH (1:10000; ab181602; 36 kDa) were got from Abcam. GAPDH was exploited to the normalizer.
2.9 Determination of RASSF1A DNA methylation
Quantitative methylation-specific PCR analysis (QMSP) was taken to examine the methylation level of RASSF1A. Genomic DNA from LC cells was obtained with the help of the QIAamp DNA mini kit (51306; QIAGEN). Total complementaryDNA was quantified utilizing the Quant-iT™ dsDNA Assay Kit (Q33120), provided by Thermo Fisher Scientific. After that, EpiTect Bisulfite Kit (59104; QIAGEN) was exploited for bisulfite modification. Utilizing an ABI 7900HT system (Thermo Fisher Scientific), the QMSP of bisulfite-transformed DNA was done with a nested, two-stage PCR method.
2.10 Chromatin immunoprecipitation (ChIP)
Beyotime provided a ChIP kit (P2078). Cells were cross-linked with 1% methanol at 37°C for 10 min and then stopped by reaction with glycine at 37°C for 5 min. The lysates were subjected to sonication to shear the DNA to a length of 200−800 bp. The sonicated sample was exposed to an anti-IgG antibody (ab37415; Abcam) or anti-CTCF antibody (ab128873; Abcam) at 4°C overnight. De-crosslinks and purification of protein/DNA complexes were conducted based on the ChIP kit. Lastly, DNA from input or immunoprecipitated samples was applied to PCR analysis.
2.11 Luciferase reporter gene analysis
We cloned the promoter sequences of RASSF1A through PCR from MRC5 embryonic lung cell DNA. RASSF1A fragments were inserted into the pGL4 luciferase reporter vector (#E6751) offered by Promega. The mutagenesis reactions were done utilizing the QuikChange mutagenesis kit (#200518) from Agilent. HEK293 cells were cotransfected with constructs and pCMV-SPORT6-CTCF expression vector (Gene Pharma) utilizing Lipo6000. After 48 h, the luminescence signal was assessed via the Dual-Lucy Assay Kit (D0010; Solarbio).
2.12 Tumor xenograft
BALB/c nude mice (male, SPF grade, weighing 18−20 g, n = 40) were acquired from Vital River Laboratory and placed in an environment with 22 ± 3°C and 50 ± 2% humidity on a 12-h light/dark cycle. All animals were offered adequate food and water. The nude mice were distributed into four groups (5 mice in each group). A549 and H1299 cells (2 × 106) transfected with RASSF1A overexpression or NC were subcutaneously inoculated into male nude mice. Tumor volumes were assessed at 7, 14, 21, 28, and 35 day. After 5 weeks, the mice were killed after anesthetizing the nude mice utilizing 0.5% sodium pentobarbital solution (50 mg/kg, P-010) offered by Supelco (Lai et al., 2020). The tumor tissues were taken out. Then, the acquired tissues were weighed, photographed, and utilized for the measurement of RASSF1A expression.
2.13 Pulmonary nodule metastasis model
Another 20 nude mice were assigned into 4 groups (5 mice each group). A549 and H1299 cells transfected with RASSF1A overexpression or NC were intravenously inoculated into the lateral tail vein of male nude mice (1 × 106 cells in 0.1 ml PBS). After 6 weeks, we killed the nude mice and resected the lung tissues. The lung tissues were taken to assess the number of metastatic pulmonary nodules.
2.14 H&E staining
We utilized 4% paraformaldehyde (G1101; Servicebio) to fix the lung tissues for 2 days. After being dehydrated and embedded, the tissues were sectioned with a thickness of 5 μm. Beyotime provided the H&E staining kit (C0105S). After being deparaffinized and rehydrated, the lung sections were exposed to hematoxylin solution for 5min. After rinsing, the sections were subjected to differentiation for 30 s. After being washed, eosin solution was employed to treat the sections for 1 min. After being rinsed, dehydrated, and permeabilized, neutral resin (abs9177; absin) was taken to seal the sections. In the end, the sections were placed in the microscope 100 times to observe the sections.
2.15 Statistics
All data were representative of triplicate independent experiments. Quantitative data were described as mean ± standard deviation and analyzed with the use of GraphPad Prism 8.0 (GraphPad Software Inc.). The differences between the two groups were assessed by a paired t-test or unpaired t-test. The comparisons among multi-groups were assessed utilizing one-way analysis of variance followed by Tukey's post hoc test. Differences were considered significant at p < .05.
3 RESULTS
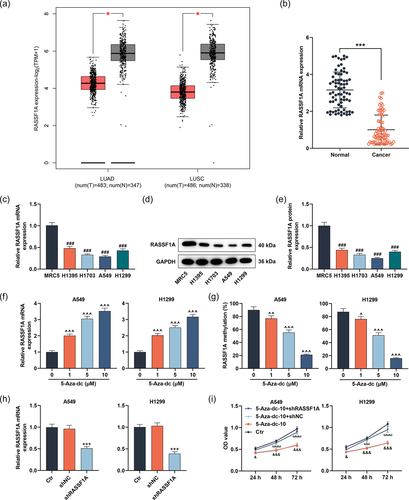
3.1 Under-expression of RASSF1A in LC partially reversed the repression of 5-Aza-dc on LC cell viability
We first evaluated the expression of RASSF1A in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) by GEPIA (http://gepia2.cancer-pku.cn/#index). RASSF1A was downregulated in LUAD and LUSC (Figure 1a, p < .05). Next, we harvested LC samples and adjacent samples from LC patients and documented that RASSF1A was under-expressed in LC tissues (Figure 1b, p < .001). The lowly expression of RASSF1A was also discovered in LC cell lines (H1395, H1703, A549, and H1299) than human embryonic lung cell line MRC5 (Figure 1c−e, p < .001). A549 cells with under-expression of RASSF1A and H1299 cells that expressed higher RASSF1A levels were singled out for the following experiments. Then, LC cells were stimulated with 1, 5, and 10 µM 5-Aza-dc for 48 h to observe RASSF1A level and the methylation level of RASSF1A in each group. 5-Aza-dc prominently enhanced the mRNA level of RASSF1A and weakened the methylation level of RASSF1A in LC cells with the increase of concentration (Figure 1f,g, p < .05). We chose 10 µM 5-Aza-dc for follow-up analysis. After that, shRASSF1A and shNC were transfected into LC cells. RASSF1A level was strongly alleviated by shRASSF1A, supporting that transfection was successful (Figure 1h, p < .001). To probe the biological roles of RASSF1A and 5-Aza-dc in LC cells, cells transfected with or without shRASSF1A or shNC were exposed to 5-Aza-dc. The weakened LC cell viability induced by 5-Aza-dc was reversed following downregulation of RASSF1A (Figure 1i, p < .05).
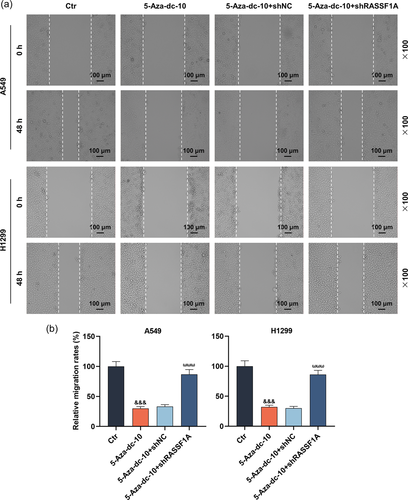
3.2 RASSF1A silencing neutralized the migration and invasion-repressing effects mediated by 5-Aza-dc in LC cells
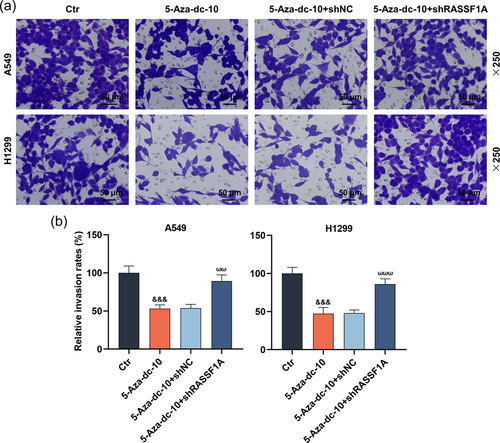
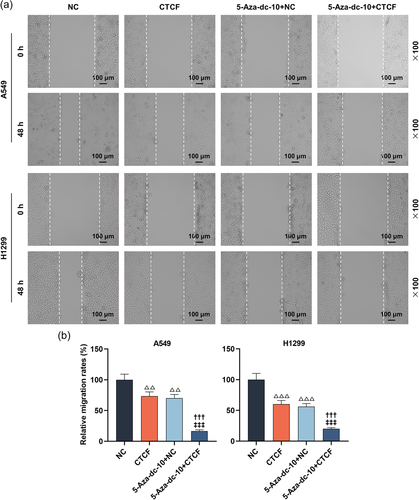
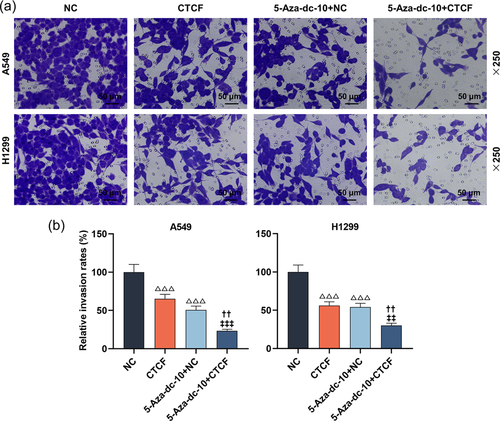
In this part, the scratch and Transwell experiments exhibited that 5-Aza-dc dramatically hindered the migration and invasion of LC cells (Figure 2a,b and Figure 3a,b, p < .001). Interestingly, RASSF1A silencing could notably reverse the functions of 5-Aza-dc on the migration and invasion of LC cells (Figure 2a,b and 3a,b, p < .001).
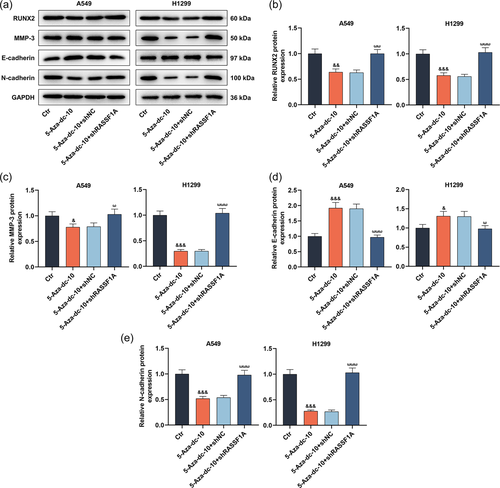
3.3 The suppression of 5-Aza-dc on epithelial-mesenchymal transition (EMT)-related factors in LC cells was reversed by RASSF1A silencing
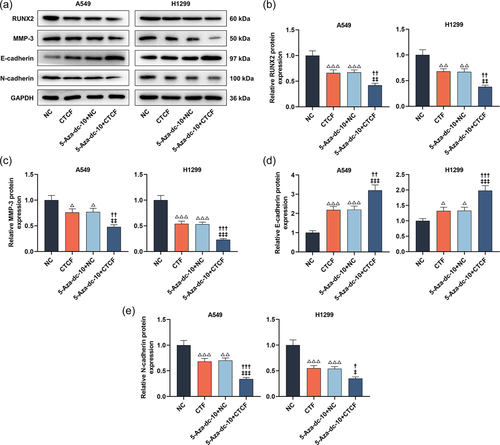
At the molecular level, we utilized Western blot analysis to assess RUNX2, MMP-3, E-cadherin, and N-cadherin levels. As manifested in Figures 4a−e, 5-Aza-dc caused the downregulation of RUNX2, MMP-3, and N-cadherin levels and the upregulation of E-cadherin level in LC cells (p < .05). However, the introduction of RASSF1A knockdown counteracted the modulation of 5-Aza-dc on RUNX2, MMP-3, E-cadherin, and N-cadherin levels in LC cells (Figure 4a−e, p < .05).
3.4 RASSF1A was regulated by the transcription factor CTCF
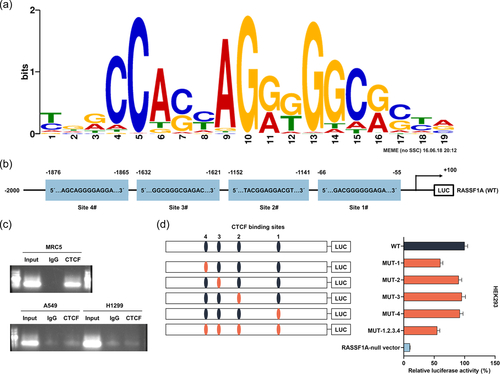
To probe the mechanism of RASSF1A, hTFtarget (http://bioinfo.life.hust.edu.cn/hTFtarget#!/) was applied to forecast the binding sites of CTCF to RASSF1A promoter (Figure 5a). We found four binding sites for the CTCF transcription factor (Figure 5b). Next, we further discovered by ChIP that CTCF bound to RASSF1A in MRC5 normal cells, but not in A549 and H1299 cancer cells (Figure 5c). The loss of CTCF binding to RASSF1A in LC cells was associated with DNA methylation. Then, the luciferase activity analysis clarified that mutation in the CTCF binding site at MUT-1, MUT-2, MUT-3, MUT-4, and all CTCF sites (MUT-1.2.3.4) within the RASSF1A promoter decreased the promoter-luciferase activity (Figure 5d).
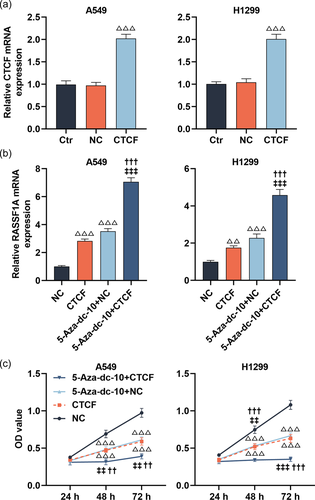
3.5 The effect of 5-Aza-dc on RASSF1A level and the malignant behaviors of LC cells were strengthened by CTCF upregulation
As described in Figure 6a, the transfection experiments were successful utilizing CTCF upregulation in LC cells; the mRNA level of CTCF was upregulated after transfection with overexpressed CTCF (p < .001). Furthermore, the RASSF1A level was apparently augmented in LC cells treated with CTCF overexpression or 5-Aza-dc (Figure 6b, p < .001). Meanwhile, CTCF overexpression further strengthened the facilitation of 5-Aza-dc on the RASSF1A level (Figure 6b, p < .001). Then, the CCK-8 assay documented that the suppression of 5-Aza-dc on the viability of LC cells at 48 and 72 h was further enhanced by CTCF upregulation (Figure 6c, p < .01). By conducting scratch and Transwell assays, we uncovered that CTCF overexpression notably weakened the migration and invasion of LC cells, and further heightened the suppression of 5-Aza-dc on the migration and invasion of LC cells (Figure 7a,b and Figure 8a,b, p < .01).
3.6 The effects of 5-Aza-dc and CTCF on RUNX2, MMP-3, E-cadherin, and N-cadherin levels of LC cells
In the next research, the Western blot analysis illuminated that LC cells transfected with CTCF overexpression plasmids greatly declined RUNX2, MMP-3, and N-cadherin levels and upregulated E-cadherin level (Figure 9a−e, p < .05). Interestingly, overexpression of CTCF further heightened the modulation of 5-Aza-dc on RUNX2, MMP-3, E-cadherin, and N-cadherin levels of LC cells (Figure 9a−e, p < .05).
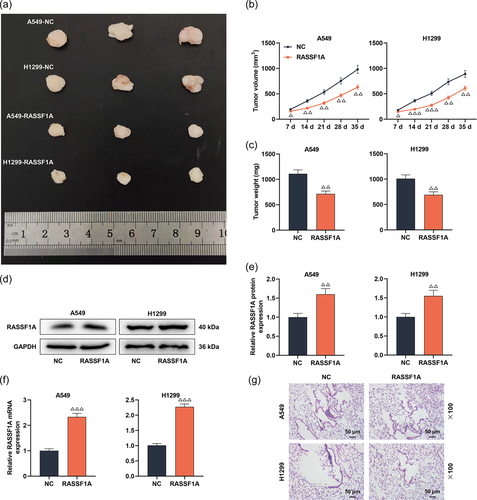
3.7 Overexpression of RASSF1A suppressed LC tumor growth and pulmonary nodule metastasis in vivo
To check the role of RASSF1A on LC cell proliferation in vivo, we constructed the xenograft tumor model. The mice in the RASSF1A group formed smaller tumor volumes and weight than that of the NC group (Figure 10a−c, p < .05). By Western blot and qRT-PCR analysis, RASSF1A was upregulated in xenografts from the RASSF1A group than that of the NC group (Figure 10d−f, p < .01). Moreover, H&E staining described that the lungs of mice injected with A549 and H1299 cells transfected with RASSF1A overexpression plasmids had fewer metastatic nodules (Figure 10g).
4 DISCUSSION
In the initiation and development of LC, DNA methylation in the promoter region is a significant cause of gene inactivation; Gene function loss can be caused by lowering genome methylation level or increasing gene promoter region methylation, leading to tumorigenesis (Chakravarthi et al., 2018). Reversing the abnormal DNA methylation in the promoter region by demethylation inhibitors may exhibit a role in repressing tumor growth (Nikbakht Dastjerdi et al., 2018). Our data uncovered that 5-Aza-dc prominently enhanced the mRNA level of RASSF1A and weakened the methylation level of RASSF1A in LC cells with the increase of concentration. This result illustrated that the methylation of RASSF1A promoter generates a considerable role in the presence and development of LC, which is consistent with previous research results on the methylation of RASSF1A promoter in cervical cancer and synovial sarcoma and is in line with expectations (Numoto et al., 2010; Yin et al., 2017).
The report pointed out that RASSF1A can be used as a new diagnostic and prognostic marker, and has important clinical reference value in assessing tumor risk, early diagnosis, and prognostic judgment (van der Weyden & Adams, 2007). The CpG island promoter hypermethylation of RASSF1A is relatively common in LC, and hypermethylation of RASSF1A is not only present in non-small cell lung cancer (NSCLC), but also in small cell LC (Dammann et al., 2001). Hypermethylation of RASSF1A promoter has been found in multiple malignancies, including LC, renal cancer, nasopharyngeal cancer, breast cancer, thyroid cancer, and other tumors (Dammann et al., 2005). A study reported the detection of RASSF1A gene methylation in cancer samples, normal samples, and plasma samples from 63 patients with LC, and found that the consistency of RASSF1A gene methylation expression in tumor tissues and plasma samples was as high as 84% (Hu et al., 2019). The expression of the RASSF1A gene transcription level was evidently related to the TNM stage and lymph node metastasis of LC, unveiling that the RASSF1A gene was involved in the development process of LC (Chen et al., 2017). Our research unveiled that RASSF1A was downregulated in LC tissues and cells. Interestingly, RASSF1A silencing could notably reverse the reduction of 5-Aza-dc on LC cell viability, migration, and invasion, indicating that methylation of RASSF1A promoter was tightly related to the biological function of LC cells, and the effective restoration of RASSF1A expression was essential for the suppression of LC.
Numerous studies proved that EMT is a key process in tumor invasion and metastasis, and RUNX2 is an important modulatory mechanism for EMT in malignant tumors and participates in the process of tumor invasion and metastasis, which has also become a hot direction of tumor metastasis research in recent years (Baumgart et al., 2007; Morita et al., 2017; Pratap et al., 2006). RUNX2 can regulate the expression of bone matrix and adhesion protein, MMP, vascular endothelial growth factor, and other proteins closely related to tumor metastasis (Baniwal et al., 2010; Yang et al., 2013). The role of RUNX2 in LC has also been explained in many studies (Tandon et al., 2012; Zheng et al., 2016). EMT is the biological process of cell transformation from a polarized epithelial phenotype to a mesenchymal fibroblast-like phenotype, which can down-regulate the level of E-cadherin epithelial protein and up-regulate the expression of N-cadherin mesenchymal protein (Lu et al., 2019). One study reported that RASSF1A impeded the invasion and metastasis of NSCLC cells via repressing YAP activation by the GEF-H1/RhoB signaling, with latent significances to defer the development of RASSF1-hypermethylated lung tumors (Dubois et al., 2016). Our study validated for the first time that 5-Aza-dc caused the downregulation of RUNX2, MMP-3, and N-cadherin levels and the upregulation of E-cadherin level in LC cells, which was reversed by RASSF1 silencing.
To probe the mechanism of RASSF1A, we further analyzed the hTFtarget database and found that there was a correlation between the transcription factor CTCF and RASSF1A; there were four sites in the promoter region of the RASSF1A gene that could bind to the common binding sites on CTCF. A report suggested that epigenetic inactivation and hypermethylation of RASSF1A in breast cancer are associated with alterations in CTCF recognition sites (Chang et al., 2010). We further discovered by ChIP that CTCF bound to RASSF1A in MRC5 normal cells, but not in A549 and H1299 cancer cells, illuminating that the loss of CTCF binding to RASSF1A in LC cells was associated with DNA methylation. Mutation in CTCF binding site at MUT-1, MUT-2, MUT-3, MUT-4, and all CTCF sites (MUT-1.2.3.4) within the RASSF1A promoter decreased the promoter luciferase activity. These findings indicated that CTCF manifested a function in the activation of the RASSF1A gene level.
CTCF is a key transcription factor that participates in the activation and inhibition of transcription via combining chromatin insulators (Gaszner & Felsenfeld, 2006). Furthermore, CTCF acts as a barrier against the spread of heterochromatin structure and DNA methylation, which exhibits a crucial function in keeping methylation-free regions (Filippova, 2008; Mukhopadhyay et al., 2004). In our study, we discovered that the effect of 5-Aza-dc on the RASSF1A level and the malignant behaviors of LC cells were strengthened by CTCF upregulation. Moreover, overexpression of CTCF further heightened the modulation of 5-Aza-dc on RUNX2, MMP-3, E-cadherin, and N-cadherin levels of LC cells.
To check the role of RASSF1A on LC cell proliferation in vivo, we constructed the xenograft tumor and pulmonary nodule metastasis models. Overexpression of RASSF1A suppressed LC tumor growth and pulmonary nodule metastasis in vivo, which further verified the results acquired from the study in vitro.
5 CONCLUSIONS
In conclusion, this paper determined that promoter methylation of tumor suppressor gene RASSF1A occurred in LC cell lines. DNA methylation blocked the modulation of RASSF1A expression by CTCF and relieved the resistance of RASSF1A to LC. The study of gene methylation may play a positive role in finding more new therapeutic targets and tumor molecular biomarkers in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was allowed via the Ethics Committee of YUE BEI PEOPLE'S HOSPITAL (SUMC-IRB-2018). No local or systemic treatment was done in these participants before the surgery. All patients signed the informed consent. All animal experiments were done in line with the Provision of Chinese Experimental Animals Administration Legislation. The animal research was ratified via the Committee of the Animal Center of Shantou University Medical College (SUMC2021-483).
Open Research
DATA AVAILABILITY STATEMENT
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.




